Comparative Studies of Noopept and Piracetam in the Treatment of Patients with Mild Cognitive Disorders in Organic Brain Diseases of Vascular and Traumatic Origin
G. G. Neznamov and E. S. Teleshova
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 108, No. 3, pp. 33-42, March, 2008.
Increasing interest in the question of the treatment of cognitive disorders arises from their significant occurrence in the population [7, 8, 39^11], which is steadily increasing as populations age, with increases in trauma and ecological problems in most of the developed countries of the world. The most frequent cognitive impairments are those resulting from brain damage of vascular, traumatic, and intoxication origin and those forming as a result of neurodegenerative diseases [7, 10, 11, 21, 25-28]. “Mild” cognitive disorders are those involving impairments of memory and other higher brain functions, beyond the age norm but not leading to social maladaptation or reaching the level characteristic of dementia [7, 15, 21, 24, 25, 40]. It should be emphasized that mild cognitive impairments are not a clearly defined nosological entity but a particular state occupying an intermediate position between normality and mild dementia [7, 10, 29], for which appropriate diagnostic criteria have been proposed, with modifications from time to time [10, 36, 39-41, 43]. As a particular degree of cognitive deficit, depending on the nosological nature of the diseases in which they develop, mild cognitive disorders are either isolated or occur in association with other psychopathologies.
Epidemiological data from the Canadian Aging Health Study (1997) and the Italian Longitudinal Aging Study (2000) indicate that cognitive disorders beyond age norms are seen in 11-17% of elderly and aged people. The risk of developing mild cognitive disorder syndrome at ages of over 65 years was 5% per year, or 19% during the four-year study period. The syndrome in most cases was a progressive state, 15% of patients per year developing dementia, a rate significantly higher than that in the overall population of elderly people. During the four-year observation period, 55-70% of cases of mild cognitive disorders were transformed into dementia [10, 29].
Epidemiological studies of people aged more than 60 years in Russia revealed mild cognitive disorders in 28.8%, including organic emotionally labile (asthenic) disorder of cerebrovascular origin in 24.4% of patients, organic personality disorders due to combined (vascular-atrophic) or complex, predominantly vascular, traumatic, and alcohol-related brain damage not reaching the stage of dementia in 3%, with long-term sequelae of craniocerebral trauma as post-concus-sional syndrome in 1.4%. The incidence of dementia among subjects aged more than 60 years reached 5.4% [7, 12].
The detection of mild cognitive disorders is extremely important from the practical point of view, because therapeutic measures can have the greatest efficacy at this stage of cerebral insufficiency [7, 10, 11, 14, 21, 28, 30, 31] and the chances of obtaining compensation once dementia has developed are significantly restricted. The use of agents with nootropic properties is recognized as one of the major approaches to the appropriate pathogenetic treatment of diseases due to organic brain damage, including those involving cognitive deficit [1, 2, 4, 10, 11, 13, 16, 23, 31], because of their positive influences on higher integrative brain functions, plastic processes in the central nervous system, energy metabolism, and the resistance of the brain to a variety of unfavorable factors [2, 4-6, 9, 13, 31, 33].
The active search for new nootropic substances has in recent years led to the appearance of ever more agents of this group, with different chemical structures, different mechanisms of action, and different spectra of pharmacological activity, for which positive influences on intellectual and memory functions are only one of the components, combined with stimulatory, anxiolytic, antidepressant, and other actions [1, 5, 6, 13, 16, 17, 38].
The present study was performed in the framework of a multicenter trial addressing clinical studies of the therapeutic efficacy and safety of the fundamentally new nootropic agent Noopept (N-phenylacetyl-L-prolylglycine ethyl ester), developed at the Zakusov Research Institute of Pharmacology, Russian Academy of Medical Sciences [9, 19, 34, 38, 44]. The present study included comparison of Noopept with piracetam. These substances were used for the treatment of patients with mild cognitive disorders in organic brain diseases of vascular and traumatic origin.
MATERIALS AND METHODS
A randomized comparative clinical trial of Noopept and piracetam was performed in two groups of patients: 37 with CNS diseases of vascular origin (aged over 50 years) and 16 patients with post-traumatic CNS damage (aged 18-60 years); a total of 53 patients was studied.
Inclusion criteria were: presence of organic emotionally labile (asthenic) disorder (F06.6) developing in association with chronic cerebrovascular insufficiency, vascular brain disease, and post-concussional syndrome (F07.2) arising at the late stages of brain trauma with cognitive and memory disorders [15]; MMSE scores for cognitive functions no greater than 27 points; absence of concomitant somatic or neurological diseases in exacerbation requiring ongoing pharmacotherapy. Patients with psycho-organic diseases in primary degenerative cerebral diseases were excluded, as were those with moderate and severe dementia, states of psychomotor excitation, delusional and hallucinatory symptomatology, delirium, confusion, moderate and severe depressive disorders, signs of chronic alcoholism, medication and drug addiction, and pregnancy, and breast-feeding; re-entry into the study was not permitted.
The duration of patients’ involvement in the study included the pre-treatment screening period (seven days) and the treatment period (56 days).
Noopept was used at a daily dose of 20 mg (as two doses) and piracetam at a daily dose of 1200 mg (as three doses). Of 37 patients with organic emotionally labile (asthenic) disorder, 21 received Noopept and 16 received piracetam; of 16 with post-concussional syndrome, 10 received Noopept and six received piracetam. Treatment was given as monotherapy in out-patient conditions with observations of ethical requirements in accord with the Helsinki Declaration and after patients provided written consent.
Investigation of patients before and during the trial included the following methods.
1) A scale assessing the severity of symptoms based on the Unified Assessment of Clinical-Pharmacological Actions of Psychotropic Agents in Patients with Organic Disorders [3], which provides objective quantitative data on the therapeutic dynamics of psychopathological symptomatology and the characteristics of the psychotropic actions of agents.
2) The Mini Mental State Examination (MMSE), which consists of a battery of neuropsychological tests evaluating cognitive functions in points: attention, memory, gnosis, speech, praxis, and counting [32].
3) The Brief Cognitive Rating Scale (BCRS), which provides assessment of the severities of the individual components of cognitive impairments [42].
4) The Cognitive Capacity Screening Examination (CCSE), which consists of a battery of tests assessing cognitive functions: orientation, memory, counting, and the abilities to infer and to group objects [35].
5) The Clinical Global Impression scale, which provides quantitative assessments of the therapeutic efficacies of agents, along with tolerance and safety, using investigations of measures of disease severity, the level of “overall improvement,” the therapeutic effect, and the presence and severity of side effects during treatment [37].
6) Laboratory analyses consisting of general blood and urine tests, including biochemical investigations (AST, ALT).
7) ECG traces.
All data were assessed statistically [8, 20, 22] using the computer program PRINN, which is a “decision-taking system” allowing expression of the combined influences of variables on overall effects with evaluation of information value and significance, which yields more objective comparative assessments (an integral measure of utility and advantages) of the alternatives under consideration. Comparison of sets was performed using the Mann-Whitney test; pre-and post-treatment observations were compared using the Wilcoxon test; differences within groups were compared using Fisher’s exact test (two-tailed version). These tests were run on Statistica.
RESULTS AND DISCUSSION
A total of 41 patients completed their 56-day treatment courses. The trial was terminated before completion in eight patients with organic emotionally labile (asthenic) disorder. These included four patients receiving Noopept (one moved house and was not available for further studies, two had increases in arterial blood pressure and required hypotensive treatment, and one withdrew because of lack of benefit) and four patients receiving piracetam (withdrawal was because of side effects). In post-concussional syndrome, treatment was terminated in four patients receiving piracetam (one because of side effects, three because of lack of efficacy).
There were no significant differences between the groups of patients treated with Noopept and piracetam in terms of demographic characteristics, disease duration, concomitant treatment, and various other parameters. The mental state of patients with organic emotionally labile (asthenic) disorder of vascular origin and post-concussional syndrome was defined by psycho-organic syndrome and a combination of memory-intellectual impairments (shortterm and long-term memory impairment, some reduction in the levels of judgement and inference abilities, some manifestations of acalculia) and neurosis-like disorders -asthenic, anxious, hypothymic, and sleep disturbance variants. A predominance of weakness, listlessness, increased fatigue, situational anxiety, anxious apprehension, marked emotional lability, hypothymia, tearfulness, weak-willed reactions, affective induction, meteoropathy, arterial blood pressure lability, headaches, and dizziness was more characteristic of patients with vascular diseases. Patients with
craniocerebral trauma more often showed a combination of asthenic and emotional-hyperesthetic disorders with increased irritability and conflict; mood was often dysthymic, with a dysphoric tone; cognitive disorders in these patients were generally restricted to deterioration of shortterm memory.
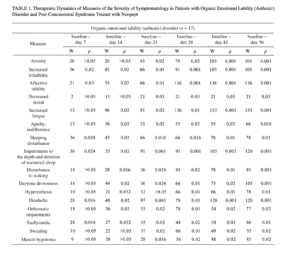
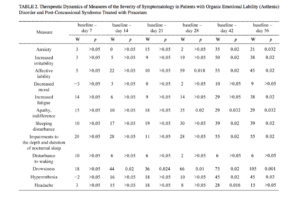
The therapeutic actions of the study agents in our patients consisted of decreases in neurosis-like symptomatology and cognitive disorders. On the symptom severity assessment scale, the effects of Noopept in patients with organic emotionally labile (asthenic) disorder of vascular origin were apparent from the first week of treatment, with reductions in increased irritability and affective lability and, probably, weakness-associated emotional tension, with improvements in going to sleep and the depth and duration of nocturnal sleep (Table 1). From the second week of treatment, there were reductions in increased fatigue, listlessness, weakness, tiring, apathy, and daytime drowsiness; from the
third week, there were reductions in anxiety, depressed mood, and hyperesthesia. These changes were accompanied by the autonomic-normalizing action of Noopept, with decreases in the severity of headaches and somato-autonom-ic symptomatology. The therapeutic dynamics of these neurosis-like symptoms are consistent with previous data [18] showing that the spectrum of action of Noopept includes anxiolytic and psychostimulatory effects.
Patients with post-concussional syndrome (see Table 1) also showed effects on both emotional-hyperesthetic (increased irritability, anxiety, affective lability) and hypos-thenic (increased fatigue, listlessness, weakness, tiring, apathy) changes. Statistically significant decreases in disorders were seen somewhat later than in patients with organic emotionally labile (asthenic) disorder of vascular origin, i.e., from treatment weeks 3-4. Noopept had no effect on abnormalities of going to sleep, impairments to the depth and duration of sleep, waking disturbances, or daytime drowsiness in patients with post-concussional syndrome.
In patients with organic emotionally labile (asthenic) disorder and post-concussional syndrome, Noopept also produced, at 7-14 days of treatment, positive changes in somatoneurological signs (see Table 1). During Noopept treatment, virtually all patients with organic emotionally labile (asthenic) disorder of vascular origin showed improvements in measures reflecting the autonomic-normalizing effect of Noopept (headaches, orthostatic abnormalities, tachycardia, sweating, muscle hypotonia; p < 0.05), except for signs which were not informative for this category of patients (mouth dryness, vasomotor lability, pain in various body parts, nausea, hypotonia, paroxysmal autonomic abnormalities). In patients with post-concussional syndrome, unlike this group of patients, Noopept had no effect on signs such as muscle hypotonia and sweating. It was only at the end of Noopept treatment courses that there was any significant decrease in the severity of headache.
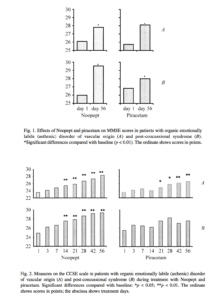
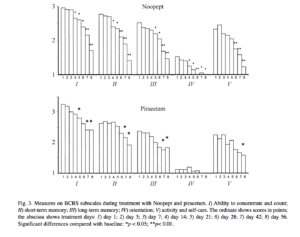
the gradual appearance of the actions of Noopept, with decreases in impairments to attention and memory apparent both in the results of specific testing and social interactions and the effectiveness of daily activities. The effects of Noopept on impairments to cognitive functions in patients with organic emotionally labile (asthenic) disorder and post-traumatic impairments were apparent as statistically significant reductions in scores on the MMSE, BPRS, and CCSE scales, starting from the third week of treatment (Figs. 1-3). Significant positive changes, identifying the nootropic effects of Noopept, starting from 2-3 weeks of
treatment, were seen in all BCRS measures in patients with organic emotionally labile (asthenic) disorder of vascular origin; in patients with post-concussional syndrome, improvements were seen in the ability to concentrate and count and in short-term memory for ongoing events and long-term memory for the past (see Fig. 3).
Thus, the use of Noopept in patients with mild cognitive disorders in organic CNS lesions of vascular origin and post-concussional syndrome led to significant reductions in all symptomatology, with decreased manifestations of both cognitive deficit and neurosis-like disorders. The agent had
Along with effects on neurosis-like symptomatology, the period from 2-4 weeks of treatment was associated with nootropic effects, psycho stimulatory actions including anti-asthenic and anxiolytic effects, positive effects on hypothymic disorders and sleep, and autonomic-normalizing actions.
The actions of piracetam differed in being more variable. In terms of the extent of the therapeutic effects of piracetam and the rate on onset, individual patients showed significant characteristics and differences.
Four patients (18%) showed reductions in fatigue, weakness, and daytime drowsiness from the first week; patients became more active, speech became quicker, and optimism regarding the present and future appeared, along with self-confidence. Nine patients (41%) who had psy-chopathological disorders whose structure included the combination of asthenic and hyperesthetic impairments, showed reductions in anxiety, the feeling of inner tension, anxious apprehension, and restlessness from the first weeks of treatment. The patients became calmer, self-confident, and optimistic. The reported decreases in irritability, previously occurring in response to minor causes. These changes were accompanied by improvements in the performance of domestic duties which had previously caused fatigue, tiredness, weakness, and irritability. In five patients (22.7%) with clear signs of the state of anxiety, irritability, emotional extravagance, and irascibility, signs of the hyperesthetic
symptom complex increased. Patients showed more frequent appearance of irritable reactions and irascibility and worsening of sleep. These changes were accompanied by decreases in weakness and daytime drowsiness. Within the first days, these patients showed increases in anxiety, the feeling of inner tension, and anxious apprehension, as well as deterioration of mood. Previously existing irritability, lachrymosity, and remorse increased. Patients complained of weakness, increased indifference, and apathy. Four patients (18%) had increased arterial blood pressure through virtually the whole period of courses of treatment, along with headaches and vertigo. There were also oscillations in the severity of anxiety, increased fatigue, and irritability, which corresponded to periods of deteriorations in physical state.
From the second half of course of treatment with piracetam, virtually all patients showed decreases in forgetfulness and improvements in the concentration of attention. Patients answered questions more quickly, chose the appropriate words, had easier recall of characters from books and film stars, and became better oriented to daily activities.
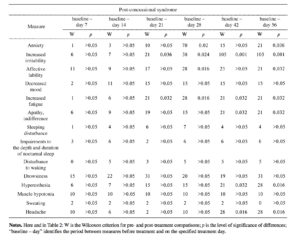
Because of the marked differences in the dynamics in individual patients, statistically significant changes in neuro-sis-like symptoms due to piracetam (Table 2) differed from those during Noopept treatment in that the development of
the therapeutic effect was slower. Significant reductions in most measures (anxiety, increased irritability, affective lability, apathy, increased fatigue, sleep disturbances) at 28-42 days and subsequently appear to provide evidence that piracetam had less marked antiasthenic and, particularly, anxiolytic effects than Noopept. The autonomic-normalizing actions of piracetam were also less marked.
Analysis of the effects of piracetam on cognitive scales in the groups of patients studied here are supported by numerous data showing that piracetam has a marked nootropic effect. In patients with organic emotionally labile (asthenic) disorder of vascular origin, there were statistically significant improvements (p < 0.05) in the MMSE scales (attention, memory, speech, gnosis, praxis), BPRS, and CCSE scales) (see Figs. 1-3). Piracetam had a positive influence on all measures of the Brief Cognitive Rating Scale, with the exception of orientation (see Fig. 3). Because of the premature termination due to side effects or inefficacy of piracetam monotherapy in most patients with post-concussional syndrome, it was very difficult to produce a dynamic assessment of the effects of this agent on individual measures in patients of this group, so an appropriate comparison in this part of the study was only possible for the group of patients with organic emotionally labile (asthenic) disorder of vascular origin. Comparison of Noopept and piracetam in patients with organic emotionally
labile (asthenic) disorder (Mann-Whitney test, comparison of two sets) using the MMSE, BPRS, and CCSE scales revealed no significant differences. Thus, the data obtained here indicate that the extents of the nootropic actions of Noopept at a dose of 20 mg/day and piracetam at a dose of 1200 mg/day were comparable.
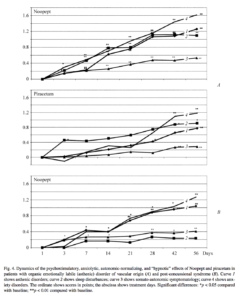
Figure 4 shows data on changes in the main clinical-pharmacological effects of Noopept and piracetam. Treatment with Noopept produced a clear balance between the activating and tranquilizing actions, an autonomic-normalizing effect, and a positive influence on sleep disturbances. On piracetam treatment, the activating effect dominated over the insignificant anxiolytic effect, while the autonomic-normalizing effect was minimal and the positive effect on sleep was barely seen.
Analysis of the therapeutic efficacies of courses of Noopept were performed using the CGI in all patients in the trial using the general improvement subscale. In patients completing all stages of the study, efficacy was evaluated at the last visit, with assessment on the last treatment day in patients prematurely finishing treatment. Noopept was found to have high efficacy in the treatment of patients with organic emotionally labile (asthenic) disorder of vascular origin, with marked improvement in 47.6% of patients and significant improvements in 42.9%; there was no improvement in 9.5% of patients. Noopept was also highly effective
Fig. 4. Dynamics of the psychostimulatory, anxiolytic, autonomic-normalizing, and “hypnotic” effects of Noopept and piracetam in patients with organic emotionally labile (asthenic) disorder of vascular origin (A) and post-concussional syndrome (B). Curve 1 shows asthenic disorders; curve 2 shows sleep disturbances; curve 3 shows somato-autonomic symptomatology; curve 4 shows anxiety disorders. The ordinate shows scores in points; the abscissa shows treatment days. Significant differences: *p < 0.05 compared with baseline; **p < 0.01 compared with baseline.
in patients with post-concussional syndrome, with marked improvement in 70%, significant improvement in 10%, and minor improvement in 20% of patients.
Assessment of the efficacy of piracetam in the patients showed that the therapeutic efficacy in patients with organic emotionally labile (asthenic) disorder of vascular origin was as follows: there were marked improvements in 25% of patients, significant improvements in 25%, minor improve
ments in 37.5%, and insignificant deterioration in 12.5%. Therapeutic efficacy in patients with post-concussional syndrome was as follows: significant improvements were seen in 16.7% of patients, with minor improvements in 50%, and no effect in 33.3%. These data provide evidence that piracetam has quite high efficacy in organic disorders of vascular origin and low efficacy in patients with post-concussional syndrome.
Comparison of the efficacies of Noopept and piracetam revealed differences on assessment of disease severity at the moment of completing treatment on the CGI scale in patients with organic emotionally labile (asthenic) disorder of vascular origin. The significant interval for the actual difference in proportions ranged from 0.07 to 0.69, i.e., there was a 95% probability that Noopept treatment would yield higher values in patients with organic emotionally labile (asthenic) disorder of vascular origin than treatment with piracetam, by 7-69%.
Analysis of treatment results in patients given Noopept and piracetam using the general improvement subscale of the CGI scale was addressed by classifying treatment efficacy into high and low ranges. High efficacy included patients with marked improvements and significant improvements; low efficacy included patients with minor
improvements, lack of efficacy, and deterioration. Analysis of the efficacy of Noopept and piracetam using the %2 test provided evidence that the efficacy of Noopept was significantly greater than that of piracetam in patients with organic emotionally labile (asthenic) disorder of vascular origin (p < 0.001) and post-concussional syndrome (p < 0.001).
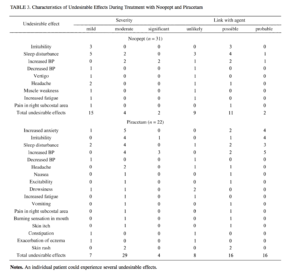
The standard methodology was used to assess undesirable effects and side effects by formal recording of all negative changes in patients’ state during treatment (Table 3), with analysis of their possible connections with treatment with the two agents. The undesirable effects noted on use of Noopept included increased sleep disturbance, irritability, and increased blood pressure. However, only sleep disturbance and increased blood pressure could be adequately linked to the agent in only two patients. In most cases, undesirable effects on treatment with Noopept were tran-
sient and of insignificant severity. The use of piracetam was associated with a greater frequency, severity, and spectrum of undesirable effects in the patients, which were dominated by effects linked with the excessive stimulatory action of the agent, along with somato-autonomic impairments.
As compared with piracetam, the use of Noopept was associated with a 1.8-fold lower level of undesirable effects, a risk of developing undesirable effects in patients of 22% to 76%, and a probable link between undesirable effects and use of the agent of 13-57% (calculations using the computer program PRINN, which allows expression of the combined influences of variables on the total effect with consideration of their information value and significance, with a more objective evaluation – an integral measure of usefulness and advantages – of the alternative being tested [8, 22]).
The results of the present trial of Noopept and piracetam supported previous data [25] on the characteristics of the actions of Noopept, which form a combination of nootropic, mild psychostimulatory, and anxiolytic effects. Noopept was found to produce positive therapeutic influences on cognitive deficit and neurosis-like symptomatology in patients with emotional lability (asthenic) disorder of vascular origin and post-concussional syndrome. There were no significant differences in the extents of the nootropic effects of Noopept and piracetam. It should only be noted that unlike the balanced profile of the therapeutic activity of Noopept, the action of piracetam included a predominance of psychostimulatory effects, which are undesirable in the treatment of patients with mild cognitive disorders. Noopept had an advantage over piracetam in terms of therapeutic efficacy and safety.
These results overall provide evidence that patients with psychoorganic disorders (in which the clinical picture is determined by the combination of mild cognitive disorders and neurosis-like disorders) can achieve better therapeutic outcomes by using Noopept.
REFERENCES
1. A. S. Avedisova, R. V. Akhankin, V. I. Akhapkina, et al., ‘Analysis of non-Russian studies of nootropic agents (using piracetam as an example), Ros. Psikhiat. Zh., 1, 57-63 (2001).
2. G. Ya. Avrutskii and A. I. Niss, Pharmacology of the Nootropes (Experimental and Clinical Studies) [in Russian], Moscow (1989),
pp. 112-118.
3. Yu. A. Aleksandrovskii, G. M. Rudenko, G. G. Neznamov, et al., A Unified System for Evaluating the Clinical-Pharmacological Actions of Psychotropic Agents in Patients with Borderline Neuropsychiatric Disorders [in Russian], Moscow (1984).
4. Yu. A. Aleksandrovskii, Psychopharmacotherapy [in Russian], Moscow (2005).
5. A. V. Valdman and T. A. Voronina, Pharmacology of the Nootropes (Experimental and Clinical Studies). Collected Studies [in Russian], Moscow (1989).
6. T. A. Voronina and S. B. Seredenin, “Nootropic agents, advances and new problems,” Eksperim. Klin. Farmakol., 61, No. 4, 3-9 (1998).
7. S. I. Gavrilova, The Pharmacotherapy of Alzheimer’s Disease [in Russian], Puls, Moscow (2003).
8. G. Giants, Medical and Biological Statistics [Russian translation from English], Moscow (1999).
9. T. A. Gudasheva, R. U. Ostrovskaya, S. S. Trofimov, et al., “Peptide analogs of piracetam as ligands of presumptive nootrope receptors,” Khim. -Farm. Zh., 11, 1322-1329 (1985).
10. I. V. Lamulin, Mild Cognitive Impairments. Methodological Guidelines for Doctors [in Russian], RKI Severo Press, Moscow (2004).
11. V. V. Zakharov and N. N. Yakhno, Memory Disorders, GEOTAR-MED, Moscow (2003).
12. Ya. B. Kalyn’, Mental Health of the Population of Elderly and Aged People (A Clinical-Epidemiological Study) [in Russian], Author’s Abstract of Doctoral Thesis in Medical Sciences, Moscow (2001).
13. G. V. Kovalev, Nootropic Substances [in Russian], Volgograd (1990).
14. O. S. Levin, “Diagnosis and treatment of moderate cognitive impairments in the elderly,” Zh. Nevrol. Psikhiat., 8, 42^-9 (2006).
15. International Statistical Classification of Diseases and Health-Related Problems (ICD-10) [Russian translation], WHO, Geneva (1995).
16. S. N. Mosolov, Basic Psychopharmacology [in Russian], Vostok, Moscow (1996).
17. G. G. Neznamov, S. A. Syunyakov, I. A. Davydova, and E. S. Teleshova, “The ‘fast’ and ‘slow’ components of the psychotropic actions of agents with nootropic properties,” Zh. Nevrol. Psikhiat., 100, No. 6, 33-37 (2000).
18. G. G. Neznamov, E. S. Teleshova, S. A. Syunyakov, et al., “Treatment of mental disorders in neurology. Clinical studies of the new peptide agent Noopept in patients with psychoorganic disorders,” Consilium Medicum (Neurology/Mental Disorders), 9, No. 2, 10-15 (2007).
19. R. U. Ostrovskaya, T. A. Gudasheva, T. A. Voronina, and S. B. Seredenin, “The original nootropic and neuroprotective dipeptide Noopept (GVS-111),” Eksper. Klin. Farmakol., 65, No. 5, 66-72 (2002).
20. S. A. Piyavskii, Numerical Decision-Taking Methods in Computerized Methods in Technical Creativity in Construction [in Russian], ASV, Moscow (1994).
21. Handbook of Psychiatry [in Russian], A. S. Tiganov (ed.), Medi-tsina, Moscow (1999), Vol. 2.
22. V. I. Sergienko and I. B. Bondareva, Mathematical Statistics in Clinical Trials [in Russian], Moscow (2001).
23. A. A. Skoromets, Neurotransmitters in Brain Aging: the Key to Understanding Memory and Attention Disorders [in Russian], Moscow (2005).
24. E. Amaiz and O. Almkvist, “Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease,” Acta Neurol. Scand., 107, Supplement 179, 34-41 (2003).
25. D. A. Bennett, “Mild cognitive impairment,” Clin. Geriat. Med., 20, 15-25 (2004).
26. H. Braak, K. Del Tredici, and E. Braak, “Spectrum of pathology,” in: Mild Cognitive Impairment, R. C. Petersen (ed.), Oxford University Press (2003), pp. 149-189.
27. C. E. Coffey, W. E. Wilkinson, I. A. Parashos, et al., “Quantitative cerebral anatomy of the aging human brain,” Neurology, 42, 527-536 (1992).
28. H. C. Comijs, M. G. Dik, D. J. H. Deeg, et al., “The course of cognitive decline in older patients,” Dement. Geriat. Cogn. Dis., 17, 136-142 (2004).
29. E. Daly, D. Zaitchik, M. Copeland, et al., Clinical Predictors of Conversion to Alzheimer’s Disease, B. Vellas et al. (eds.), Serdi Publisher, Paris (2001), Vol. 5, pp. 44^19.
30. W. Danysz, C. G. Parsons, H.-J. Mobius, et al., “Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease – A unified glutamatergic hypothesis on the mechanism of action,” Neurotox. Res., 2, 85-97 (2000).
31. D. De Wied, Neurochemical and Psychopharmacological Approaches to Cognitive Entracers, Kyoto (1995).
32. M. F. Folstein, S. E. Folstein, and P. R. McHugh, J. Psychiat. Res., 12, No. 3, 189-198 (1975).
33. C. Giurgea, “Vers une pharmacologie de l’activite integrative du cerveau. Tentative du concept nootrop en psychopharmacologie,” Actual Pharmacol., 25, 115-157 (1972).
34. T. Gudasheva, T. Voronina, R. Ostrovskaya, et al., “Synthesis and antiamnestic activity of a series of N-acylprolyl-containing dipeptides,” Eur. J. Med. Chem., 31, 151-157 (1996).
35. J. W. Jacobs, M. R. Bernhard, F. Delgado, and J. J. Strain, Ann. Int. Med., 86, 40-46 (1977).
36. D. S. Knopman, B. F. Boeve, and R. C. Petersen, “Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia,” Mayo Clinic Proc., 78, 1290-1308 (2003).
37. National Institute of Mental Health, 12-CGI. Clinical Global Impression, W. Guyo (ed.), ECDEU Assessment Manual for Psychopharmacology, Rockville, Maryland (1976), 217-222.
38. R. Ostrovskaya et al., Anxiolytic Activity of Nootropic Dipeptide Noopept, Nova Sci. Publ. (2006), Vol. 10, pp. 1-10.
39. K. Palmer, L. Backman, B. Winblad, and L. Fratiglioni, “Detection of Alzheimer’s disease and dementia in the preclinical phase: population based cohort study,” Brit. Med. J., 326, 245-249 (2003).
40. R. S. Petersen, G. E. Smith, S. C. Waring, et al., “Mild cognitive impairment: clinical characterization and outcome,” Arch. Neurol., 56, 303-308 (1999).
41. R. S. Petersen, “Mild cognitive impairment: transition from aging to Alzheimer’s disease,” in: Advances of Etiology, Pathogenesis, and Therapeutics, K. Igbal et al. (eds.), Wiley, Chichester, etc. (2001), pp. 140-151.
42. B. Reisberg, S. H. Ferris, et al., Psychopharm. Bull., 19,47-50 (1983).
43. K. Ritchie, S. Artero, and J. Touchon, “Classification criteria for mild cognitive impairment. A population-based validation study,” Neurology, 56, 37-42 (2001).
44. S. Seredenin, T. Voronina, T. Gudasheva, R. Ostrovskaya, et al., “Biologically active N-acylprolyldipeptides having antiamnestic, antihypoxic, and anorexigenic effects,” US Patent No. 5439930 (1995).