Piracetam: A Review of Pharmacological Properties and Clinical Uses
Bengt Winblad
Karolinska Institutet, Stockholm, Sweden
Keywords: Cognitive disorders — Cortical myoclonus — Dementia — Dyslexia — GABA derivatives — Membrane fluidity — Neurotransmitters — Piracetam — Sickle cell anemia — Vertigo.
ABSTRACT
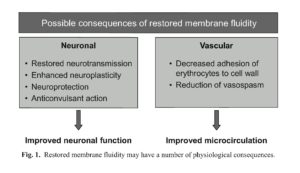
Piracetam, a derivative of the neurotransmitter y-aminobutyric acid (GABA), has a variety of physiological effects that may result, at least in part, from the restoration of cell membrane fluidity. At a neuronal level, piracetam modulates neurotransmission in a range of transmitter systems (including cholinergic and glutamatergic), has neuroprotective and anticonvulsant properties, and improves neuroplasticity. At a vascular level, it appears to reduce erythrocyte adhesion to vascular endothelium, hinder vasospasm, and facilitate microcirculation. This diverse range of physiological effects is consistent with its use in a range of clinical indications. Its efficacy is documented in cognitive disorders and dementia, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia. While high doses are sometimes necessary, piracetam is well tolerated.
INTRODUCTION
Piracetam is a cyclic derivative of the neurotransmitter y-aminobutyric acid (GABA), originally marketed in 1971 by UCB Pharma. It was the first “nootropic” drug (25), an agent that acts on cognitive function without causing sedation or stimulation. While the mechanisms of action of piracetam have yet to be fully elucidated, it influences neuronal and vascular functions. Furthermore, vascular effects are peripheral as well as central, meaning that the clinical benefit of piracetam goes beyond its nootropic features. Indeed, piracetam is now indicated for use in vertigo, dyslexia, cortical myoclonus and sickle cell anemia in addition to age-related cognitive disorders. As piracetam has been available for
Address correspondence and reprint requests to: Dr. Bengt Winblad, Karolinska Institutet, Neurotec, Hud-dinge, University Hospital B 84, S-14186 Stockholm, Sweden. E-mail: bengt.winblad@neurotec.ki.se.
over 30 years, the purpose of this article is to provide a review of its possible mechanism of action, pharmacological properties and clinical uses.
POSSIBLE MECHANISM OF ACTION:
THE MEMBRANE HYPOTHESIS
Although piracetam is a derivative of GABA, its mechanism of action appears to be unrelated to the properties of this neurotransmitter. While the exact mode of action of piracetam is a matter of debate, there is increasing evidence that its underlying effect is to restore cell membrane fluidity, so it is on this theory that this manuscript will focus. The restoration of membrane fluidity is neither cell nor organ specific, and might, therefore, explain the diverse physiological effects of piracetam.
Cell membranes comprise a bilayer of lipid molecules interspersed with protein molecules. These membranes are fluid structures in which the molecules comprising the membrane can diffuse while maintaining this overall arrangement. Membrane fluidity is believed to be important for a number of activities including membrane transport, enzyme activity, chemical secretion, and receptor binding and stimulation (2,11).
The interaction of piracetam with cell membranes was first reported in a study in which piracetam partially prevented alcohol-related changes in a synthetic phosphatylcholine monolayer (21). This observation was subsequently supported by a magnetic resonance spectroscopy study involving artificial membranes, which showed piracetam molecules surrounding the polar head group of phospholipids. The resultant mobile drug-lipid complexes are thought to induce the reorganization of lipids, which may influence membrane function and fluidity (66).
The interaction of piracetam with membranes has also been reported in another, recent, in vitro study investigating the toxic effect of amyloid peptide aggregates on neuronal membranes (47). Amyloid peptide was shown to cause lipid disorganization within the cell membranes, and incubation with piracetam significantly decreased the destabilizing effects of the amyloid peptide. The authors proposed that this effect could result from the interaction between piracetam and phospholipid head groups within the cell membranes. For example, this interaction might increase the area occupied by the head group, which would induce a positive curvature of the membrane. Thus, piracetam may counteract the negative curvature induced by fusogenic peptides, and, therefore, decrease the likelihood of membrane fusion.
Studies have also demonstrated that piracetam influences membrane fluidity, particularly when normal fluidity is compromised, as is often seen during aging (56,74). Several in vitro studies have assessed fluidity during piracetam treatment using anisotropy of membrane-bound DPH (1,6-diphenyl-1,3,5-hexatriene). Incubation with piracetam restored fluidity in brain membranes of elderly mice with diminished fluidity but had no effect on brain membranes of younger mice with normal fluidity (56). Similarly, in other studies using in vitro anisotropy techniques, fluidity was restored in the membranes of aged rat and aged human brains following incubation with piracetam (56). Similar effects were observed in hippocampal membranes from patients with Alzheimer’s disease (19). It has also been shown that 8 weeks of treatment of aged rats with piracetam (300 mg/kg daily) significantly restored membrane fluidity in the frontal cortex, hippocampus and striatum. This improvement of fluidity coincided with significantly improved avoidance learning (56). No effect of piracetam on learning or membrane fluidity was found in young rats receiving piracetam.
The effect of piracetam on membrane fluidity is not limited to brain membranes, however. In a study reported by Muller and colleagues (55), incubation with piracetam (1.0 mmol/L) significantly improved fluidity of platelet membranes (as measured using DPH anisotropy techniques) in samples from elderly but not young humans. Further effects of piracetam on membrane fluidity may be revealed through the use of alternative fluorescence probes; DPH anisotropy will only reveal changes in hydrocarbon core fluidity that are sensitive to DPH (55).
Numerous neuronal and vascular effects of piracetam have been described (Fig. 1) and will be discussed in the following sections. While conclusive data are not yet available, it is possible that changes in membrane fluidity could explain, at least in part, many of these physiological observations. For example, membrane fluidity is known to affect several membrane activities including membrane transport, chemical secretion, and receptor binding and stimulation (2,11).
PHARMACOLOGICAL EFFECTS OF PIRACETAM
Neuronal Effects
Effects on neurotransmission
Piracetam has important effects on neurotransmission that are not limited to any one type of neurotransmitter. It has been shown to influence cholinergic (54,67,80,89), seroto-ninergic (84), noradrenergic (61), and glutamatergic (9) systems. The modulation of these systems by piracetam does not result from direct receptor agonism or antagonism (piracetam has no affinity for these receptors; K{> 10 pM) (28). Instead, piracetam appears to increase the number of postsynaptic receptors and/or restore the function of these receptors. The membrane hypothesis of piracetam’s action would predict that neurotransmitter function is affected by membrane fluidity because the fluidity of the membrane impacts
on the proteins embedded within the membrane. Neurotransmitters bind to these proteins modulating the flow of ions and other chemicals in and out of the cell and, therefore, influencing cell signaling. If fluidity is modified (e.g., by piracetam), neurotransmitter action, and thus cell signaling, would be affected.
The effect of piracetam on cholinergic and glutamatergic systems is likely to be particularly relevant to its clinical benefit in cognitive disorders, given the increasing evidence that dysfunction in these systems may be related to cognitive decline (77,82). Piracetam modifies hippocampal acetylcholine levels in the rat (89) and increases the population of muscarinic cholinergic receptors in the frontal cortex of aged but not young mice by up to 40% (67). Furthermore, carbachol-induced accumulation of inositol-monophosphates (a measure of muscarinic cholinergic receptor function that decreases with age) was elevated following piracetam treatment suggesting that piracetam can normalize functional deficits associated with aging (54,80). In the glutamatergic system, 14 days of treatment with piracetam has been shown to significantly increase N-methyl-D-aspartate (NMDA) receptor density in the forebrain of aging mice by approximately 20%. Furthermore, piracetam treatment normalized the age-related elevated affinity of L-glutamate for the NMDA receptor, suggesting that it can restore NMDA receptor function (9).
Neuroprotective effects
Preclinical studies have shown that piracetam appears to offer neuroprotective benefits in several circumstances. This is consistent with the suggestion that interactions between piracetam and membrane lipids may decrease the risk of membrane fusion (47). Piracetam has been shown to reduce the incidence of animal death following barbiturate overdose, and to protect against morphological changes related to long-term alcohol use (5). In alcohol-treated rats, piracetam administration is associated with a decrease in lipofuscin, a marker of neuron membrane damage (65); the volumetric density of lipofuscin granules in cerebellar Purkinje cells was 1.5 and 2.7% with and without piracetam, respectively (p<0.01). Piracetam administration initiated in rats 1 h after cortical lesions and continued twice daily for 3 weeks also reduced the extent of ischemic damage relative to placebo (90). At the end of the treatment period, animals receiving piracetam showed a 20-21 % decrease in cortical tissue in the measured area, whereas the decrease in those receiving placebo was 26-30% (between-treatment difference, p < 0.03). Despite these promising preclinical findings, clinical studies have failed to demonstrate marked benefits of piracetam in individuals following acute stroke (15,63,70,71). This may be due to the difficulties in studying the heterogeneous stroke population.
Effects on neuroplasticity
Neuroplasticity refers to the adaptation of neural circuitry through the modification and development of synaptic and neural connections. This process is heavily involved in learning and memory and has also been implicated in limiting the effects of ischemic damage and degenerative lesions. The neuroplastic effects of piracetam have been reported in two studies involving alcohol-treated rats (4,5). Alcohol consumption is associated with neuronal loss, which may be exacerbated during withdrawal. In addition to reducing withdrawal-related neuronal loss (5), piracetam has been shown to increase the number of synapses in the hippocampus by up to 20% relative to alcohol-treated or alcohol-withdrawn rats (4). This latter observation suggests that piracetam promotes neuroplasticity when neural circuits are recoverable.
Anticonvulsant effects
The anticonvulsant action of piracetam is well documented in animal studies. Administration of piracetam prior to a convulsant stimulus reduces seizure severity in rats prone to audiogenic attacks (3). Furthermore, piracetam enhances the anticonvulsant effects of car-bamazepine (31,51,52), and diazepam (43). For example, the combination of piracetam with carbamazepine protected 80%, instead of 30%, of animals from electroshock-induced convulsions (/?< 0.001) (51). The anticonvulsant mechanism of piracetam is not fully understood, but may be related to its effects on neurotransmitters.
Vascular Effects
Effects on erythrocytes
Studies suggest that piracetam exerts a number of effects on erythrocytes, such as decreased adhesion to endothelium (57). These effects are likely to facilitate movement of erythrocytes through the circulation.
Reduced erythrocyte adhesion to the endothelial wall following piracetam administration was reported by Nalbandian and colleagues (57). Blood taken from healthy individuals and from those with sickle cell anemia was incubated with saline or 1.5 mmol/L of piracetam for at least 30 min prior to additional incubation with endothelial cell culture (57). Piracetam significantly reduced adhesion to endothelial cells in both normal (normalized adherence = 1.0 and 0.88 for saline and piracetam, respectively, p < 0.02), and sickle cell preparations (normalized adherence = 1.15 and 0.84 for saline and piracetam, respectively,/? < 0.02).
Effects on blood vessels
Studies have indicated that piracetam exerts an effect on blood vessels. For example, in vitro, 2 mg/kg piracetam decreased the time taken for rabbit pial vessels to return to normal diameter following a period of induced arteriolar spasm (10.4 vs. 5.1 min for 0.02 and 2 mg/kg of piracetam, respectively) (69). A single tablet dose of piracetam (3.2, 4.8, or 9.6 g) has also been reported to stimulate prostacyclin synthesis (53). Mean 6-keto-PGFla before and 4 h after 4.8 g piracetam was 58.8 vs. 129.2 pg/mL, respectively (p = 0.02). This effect on prostacyclin synthesis is likely to contribute to the vascular effect of piracetam since prostacyclin has vasodilator as well as platelet aggregation inhibitory properties (48-50,76).
Effects on blood coagulation
Piracetam may also influence blood coagulation. In healthy humans, a single dose of piracetam (3.2 to 9.6 g) reduced plasma levels of fibrinogen and von Willebrand factor in a dose-dependent manner by up to 40% (53). Fibrinogen and von Willebrand factor are involved in hemostasis.
Effects on microcirculation
Enhanced cerebral blood flow has been reported following piracetam treatment in hypotensive cats and in humans with acute cerebral ischemia (35,73). Additionally, piracetam appears to influence microcirculation at the peripheral level. Following treatment with piracetam, renal blood flow was significantly greater in ischemically damaged rat
kidneys relative to controls (24), and, in a separate experiment, blood flow significantly increased in the cochlea of guinea pigs without any marked change in blood pressure (45).
This improved microcirculation by piracetam is likely to result from a combination of its effects on erythrocytes, blood vessels and blood coagulation. While the vascular effects of piracetam are likely to be important in all of its clinical uses, they have particular relevance to its efficacy in sickle cell anemia.
PHARMACOKINETICS
Piracetam is rapidly absorbed. Following oral administration, peak plasma concentrations in fasting subjects are achieved in approximately 30 min (27). Following a single oral dose of 3.2 g, peak concentration is typically 84 pg/mL. Oral formulations of piracetam are extensively absorbed with a bioavailability close to 100% (27). No metabolites of piracetam have yet been discovered and the drug is excreted unchanged in the urine by glomerular filtration (26). While food does not affect the extent of absorption of piracetam, it does decrease the maximal plasma concentration of the drug by 17% and prolong /max to 1.5 h. Piracetam crosses blood-brain and placental barriers and is found in all tissues, except adipose tissue. The uptake into the brain is less rapid than into the circulation, and, at nearly 8 h, half-life in cerebrospinal fluid is longer than in plasma (about 5 h) (26).
TOLERABILITY
Piracetam is remarkably well tolerated. In preclinical trials, no irreversible toxicity was reported in mice, rats or dogs receiving single oral doses of up to 10 g/kg. In a pooled analysis of 91 double-blind, placebo-controlled studies (piracetam n = 3017, placebo n = 2850), hyperkinesia, weight gain, nervousness, somnolence, depression and asthenia were slightly increased with piracetam, although the incidence of each of these events was less than 2%.
CONTRAINDICATIONS AND DRUG INTERACTIONS
Due to its renal clearance, piracetam dose should be adjusted in patients with renal insufficiency and the drug is contraindicated in patients with end-stage renal disease. Piracetam should not be prescribed to patients with cerebral hemorrhage. While reproductive studies in animals have not identified any risk to the fetus, studies in humans have not been conducted and so the use of piracetam in pregnant or lactating women should be avoided. Piracetam is neither metabolized by the liver nor bound to plasma albumin. The potential for drug-drug interactions is, therefore, low. Although piracetam enhances the anticonvulsant effects of carbamazepine (see above), no interactions with sodium valproate have been reported (6). There are no known interactions of piracetam with any other drugs.
THERAPEUTIC DOSE RANGE
The dosing of piracetam varies according to indication. For cognitive disorders and vertigo it is 2.4^1.8 g daily p.o., for dyslexia it is 3.2 g daily p.o., for cortical myoclonus it is 7.2-24.0 g daily p.o., for prophylaxis of vaso-occlusive crises in sickle cell anemia it is 160 mg/kg/day p.o., and for remission of vaso-occlusive crises it is 300 mg/kg/day i.v. in four divided doses.
CLINICAL USE
Consistent with its varied pharmacological effects, piracetam has documented benefit in a diverse range of indications.
Cognitive Disorders
Some loss of cognitive function is to be expected in the elderly. There is, however, a marked variability in the extent of this dysfunction. At one end of the scale, changes defined as normal include mild deficits in memory, perception and spatial recognition, and a reduction in cognitive speed (8,13,68,79). At the other end of the scale, a proportion of elderly people will develop dementia, with substantial cognitive dysfunction, impaired judgment, personality changes and loss of independent functioning. As the aging population grows, it becomes increasingly important to develop treatments to target the range of cognitive dysfunction in the elderly.
A number of pharmacological properties are relevant to the use of piracetam in cognitive disorders. For example, it has been suggested that age-related cognitive changes result from alterations in membrane structure and function (81). Furthermore, the cholinergic and glutamatergic neurotransmitter systems have been implicated in age-related cognitive decline (77,82), and neuroplasticity has been reported as a compensatory mechanism in response to Alzheimer’s disease pathology (42). It is, therefore, no surprise that the benefits of piracetam in a range of cognitive disorders in the elderly have been reported in several studies (7,12,33,34,38,64). In one study, 162 patients with age-associated memory impairment received piracetam 2.4 g/day, piracetam 4.8 g/day, or placebo for 3 months (38). All patients were also enrolled in a memory training program. The improvement with piracetam 4.8 g/day was significantly greater than with placebo alone on a range of memory tests including immediate (p < 0.0004), global (p < 0.002), and delayed (p < 0.04) recall. Piracetam 2.4 g/day produced a significantly greater improvement than placebo in immediate recall (p < 0.03).
The benefit of piracetam in the treatment of dementia is limited by the extent of the underlying pathology of the disease. Nevertheless, studies have shown that piracetam can improve cognition in patients with dementia (12,33,34,64). A study of 130 patients diagnosed with mild-to-moderate dementia compared the effect of piracetam (4.8 g/day) with placebo for 12 weeks on a range of psychometric and clinical assessment scales (33,34). At the end of treatment, patients had significantly greater improvements with piracetam than with placebo on all scales (p < 0.001). This study demonstrates that piracetam improves functioning on clinically relevant scales.
Longer-term treatment with piracetam may also limit cognitive deterioration. A yearlong, double-blind study assessed the effect of piracetam or placebo on the performance of 33 patients with early probable Alzheimer’s disease on 14 psychometric tests (12). Over the year, placebo-treated patients deteriorated on nine of the 14 scales whereas piracetam-treated patients deteriorated on only one scale.
Importantly, given the small samples involved in some of the above studies, two metaanalyses have also examined the effect of piracetam on individuals with age-related cognitive disorders (23,87). Both of these analyses used data from Clinical Global Impression of Change (CGIC) rating scales from various studies. The CGIC is an important measurement tool as it determines the clinical relevance of any reported symptomatic improvements. Indeed, the Committee for Proprietary Medicinal Products (CPMP) Note for Guidance on Medicinal Products in the Treatment of Alzheimer’s Disease (10) recommends that a global assessment of change such as the CGIC should be part of all clinical trials involving patients with Alzheimer’s disease as it is a way to validate results obtained in comprehensive scales or objective tests. These global measures are not sensitive to small changes that may be clinically insignificant (75); any change on the scale is clinically meaningful by definition.
The smaller meta-analysis included six randomized, placebo-controlled trials of patients (n = All) with vascular dementia, unclassified dementia, Alzheimer’s disease or cognitive impairment not fulfilling the criteria for dementia (23). The odds ratio for improvement on the CGIC in patients receiving piracetam compared with those receiving placebo was 3.47 [95% Cl: 1.29, 9.30] or 3.55 [2.45, 5.16] depending on whether a random or fixed effects model was used.
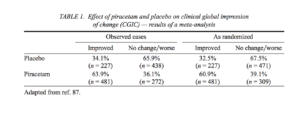
The second meta-analysis examining the effect of piracetam on the CGIC used a greater number of studies and a larger number of patients (87). This analysis incorporated both published and unpublished studies to reduce publication bias for favorable results that may overestimate the benefit of piracetam. In total, 19 studies (nine published, 10 unpublished) involving nearly 1500 patients were included. All were parallel-group, double-blind, placebo-controlled trials conducted between 1972 and 1993 involving patients aged over 50 years with age-related cognitive disorders and degenerative dementia. The meta-analysis included studies that lasted 6-52 weeks and involved piracetam doses of 2.4-8 g/day.
This meta-analysis showed that more than 60% of piracetam-treated patients improved on the CGIC compared with approximately 30% of patients receiving placebo (Table 1). These findings translate to an odds ratio for improvement in CGIC in favor of piracetam
that ranged between 2.45 (95% Cl: 1.93-3.11) and 3.35 (95% Cl: 2.70-4.17) depending on the statistical method used. Overall, the meta-analysis reported that between three and five patients would need to receive piracetam to prevent one negative outcome compared with placebo. The findings of this meta-analysis are consistent with those of the Flicker and Grimley Evans (23), but with increased robustness as demonstrated by smaller confidence intervals. While the results of these two meta-analyses were significant and indicate an overall benefit of piracetam in the treatment of cognitive disorders, additional large-scale randomized trials with piracetam in this population are desirable.
Vertigo
Vertigo is a type of dizziness that specifically refers to the illusion of movement of the self or the environment that is typically rotatory in nature. It results from disturbances in the vestibular system that can be peripheral (e.g., abnormalities in the vestibular apparatus) or central (e.g., cerebrovascular disease). Lack of sensory input from proprioceptive or visual sources can also contribute to symptoms. The clinical benefit of piracetam in vertigo may result from its effects on neurotransmission and microcirculation.
In a double-blind study of 143 elderly patients with chronic vertigo of central, peripheral or unspecified origin, piracetam 2.4 g/day was compared with placebo (72). Following 8 weeks of treatment, the number of episodes of vertigo in 2 weeks preceding evaluation had decreased by approximately nine with piracetam compared with an increase of approximately three with placebo (p < 0.05). There was no difference between the treatments in attack severity. However, between attacks, piracetam had a significantly greater beneficial effect on severity of malaise (p < 0.05) and severity of imbalance (p < 0.01) than placebo, as measured by patients on a visual analog scale.
Several other studies indicated efficacy of piracetam on vertigo of central, peripheral, or mixed origin (29,62) and also on postconcussional vertigo (1,17,30).
Cortical Myoclonus
Cortical myoclonus refers to a range of motor conditions that result from abnormal electrical activity in the sensorimotor cortex. These conditions are characterized by uncontrollable muscle movements, which may be entirely spontaneous or occur in response to certain sensory stimulants or movement. Myoclonus may also be accompanied by generalized seizures (60,86). Cortical myoclonus can be severely debilitating, limiting speech and disrupting most aspects of daily functioning. Traditional anticonvulsants typically control seizures but are less effective in managing involuntary muscle movements (86).
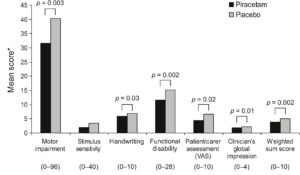
Piracetam is useful in several types of cortical myoclonus, sometimes causing marked improvements when other therapies have failed (78). Its success in treating myoclonic conditions alone or in combination with other anticonvulsants has been reported in several case reports and open trials (22,37,39,58,59,83) and in two double-blind studies (6,41). In the first of the double-blind studies, 24 patients with cortical myoclonus were included in an open-label run-in period to identify responders to piracetam and to establish clinically effective doses (6). Of these, 21 patients (87.5%) entered the double-blind, cross-over stage, during which they received piracetam (2.4-16.8 g/day) or placebo alone or in combination with existing anticonvulsant treatment. Performance on six of the seven measures of myoclonus significantly improved following piracetam relative to placebo (Fig. 2).
Fig. 2. Effect of piracetam and placebo on functional measures of cortical myoclonus. * Lower scores indicate better outcome. The possible range of scores for each scale is provided in brackets. Adapted from refs. 6,86.
These findings are consistent with those reported in a double-blind cross-over study by Koskiniemi and colleagues (41). While not all patients will respond to piracetam, they concluded that “every patient with myoclonus symptoms warrants a trial of therapy with piracetam.” Both of these studies also showed that abrupt discontinuation may induce a return to the previous clinical condition. Piracetam should, therefore, be discontinued gradually.
Dyslexia
Dyslexia can be defined as a specific difficulty in interpreting written language despite adequate intelligence and normal vision. It is often accompanied by problems with writing or spelling. Several double-blind studies have investigated piracetam in the treatment of dyslexia, and, while the findings are not entirely consistent, most report a significant effect of piracetam relative to placebo on reading speed and/or accuracy (18,44,85,88). In one study, 225 children with dyslexia, between 7 and 12 years of age, received piracetam 3.3 g/day or placebo for 36 weeks (88). At the end of treatment, piracetam significantly improved reading, compared with placebo as measured by the Gray Oral Reading Test total passage score (mean adjusted change from baseline: 7.5 and 6.0 for piracetam and placebo, respectively, p = 0.043) and by the Gilmore Oral Reading Test comprehension score (mean adjusted change from baseline: 4.3 and 2.7 for piracetam and placebo, respectively, p = 0.009). The improvement of dyslexia with piracetam is modest, however, and may take months to manifest. Best results are likely to be achieved when piracetam is combined with education programs.
Sickle Cell Anemia
Sickle cell anemia is a genetic condition resulting from abnormal or sickled hemoglobin. This abnormal hemoglobin makes erythrocytes more rigid (i.e., have reduced
deformability) and causes them to adhere more readily to the endothelial wall relative to healthy subjects (32,36,40). This erythrocyte adherence tends to occlude capillary blood flow resulting in local tissue damage and periods of pain, known as sickle cell crises. Piracetam exerts a number of effects on erythrocytes making it a potential candidate for treatment of this disease, and indeed, studies have shown its efficacy in prophylaxis and during crises (14,16,20,46).
In the largest of these studies, 101 children between 3 and 12 years of age received piracetam (300 mg/kg/day during crises and 160 mg/kg/day as prophylaxis) or placebo according to a double-blind design for up to 1 year (20). Data for children of 3 to 6 years and 7 to 12 years of age were analyzed separately. In both age groups, piracetam significantly reduced (relative to baseline) severity index (p < 0.001), number of crises {p < 0.05), number of hospitalizations (p < 0.05), and number of blood transfusions (p < 0.05). At the end of the study, in both age groups, the number of crises was significantly reduced in children receiving piracetam compared with those receiving placebo {p < 0.05). Furthermore, piracetam-treated children aged 3 to 6 years had a significantly reduced severity index compared with placebo-treated children of this age (p < 0.05).
CONCLUSION
Piracetam is a well-tolerated drug with documented clinical benefit in several conditions including age-related cognitive disorders, vertigo, cortical myoclonus, dyslexia and sickle cell anemia. Its efficacy in these conditions appears to result from a range of neuronal and vascular effects that may be related to restored membrane fluidity. Additional large-scale, controlled studies would allow researchers and clinicians to gain further insight into the clinical benefits of this interesting drug.
Acknowledgment. Many thanks to Sarah Meredith and Yannick Lambe (UCB S.A., Belgium) for their excellent support during the preparation of this article.
REFERENCES
1. Aantaa E, Meurman OH. The effect of piracetam (Nootropil, UCB-6215) upon the late symptoms of patients with head injuries. JIntMedRes 1975;3:352-355.
2. Alberts B, Bray D, Lewis J, et al. Molecular biology of the cell. Chapter 10. 3rd Edition. New York: Garland publishing Inc., 1994.
3. Benesova O. The effects of nootropic drugs on the susceptibility to audiogenic seizures in rats. Act New Super (Praha) 1980;22:192-193.
4. Brandao F, Cadete-Leite A, Andrade JP, Madeira MD, Paula-Barbosa MM. Piracetam promotes mossy fiber synaptic reorganization in rats withdrawn from alcohol. Alcohol 1996;13:239-249.
5. Brandao F, Paula-Barbosa MM, Cadete-Leite A. Piracetam impedes hippocampal neuronal loss during withdrawal after chronic alcohol intake. Alcohol 1995;12:279-288.
6. Brown P, Steiger MJ, Thompson PD, et al. Effectiveness of piracetam in cortical myoclonus. Mov Disord 1993;8:63-68.
7. Chouinard G, Annable L, Ross-Chouinard A, Olivier M, Fontaine F. Piracetam in elderly and psychiatric patients with mild diffuse cerebral impairment. Psychopharmacology 1983;81:100-106.
8. Christensen H. What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry 2001; 35:768-775.
9. Cohen SA, Muller WE. Effects of piracetam on N-methyl-D-aspartate receptor properties in the aged mouse brain. Pharmacology 1993;47:217-222.
10. Committee for Proprietary Medicinal Products (CPMP). Note for guidance on medicinal products in the treatment of Alzheimer’s disease, http://www.emea.eu.int/pdfs/human/ewp/055395en.pdf
11. Crews FT. Effects of membrane fluidity on secretion and receptor stimulation. Psychopharmacol Bull 1982; 18:135-143.
12. Croisile B, Trillet M, Fondarai J, Laurent B, Mauguiere F, Billardon M. Long-term and high-dose piracetam treatment of Alzheimer’s disease. Neurology 1993;43:301-305.
13. Cullum S, Huppert FA, McGee M, et al. Decline across different domains of cognitive function in normal ageing: Results of a longitudinal population-based study using CAMCOG. Int J Geriatr Psychiatry 2000; 15: 853-862.
14. De Araujo JT, Nero GS. Piracetam and acetamide in sickle cell disease. Lancet 1977;2:411.
15. De Deyn PP, Reuck JD, Deberdt W, Vlietinck R, Orgogozo JM. Treatment of acute ischemic stroke with piracetam. Members of the Piracetam in Acute Stroke Study (PASS) group. Stroke 1997;28:2347-2352.
16. De Melo GOS. Piracetam in sickle cell anemia. Lancet 1976;2:1139-1140.
17. Deza Bringas L. Tratamiento del sindrome subjetivo post-traumatico con piracetam. [Treatment of the subjective post-traumatic syndrome with piracetam]. Rev Neuro-Psiquiatria 1984;47:74-86.
18. Di Ianni M, Wilsher CR, Blank MS, et al. The effects of piracetam in children with dyslexia. J Clin Psychopharmacol 1985;5:272-278.
19. Eckert GP, Cairns NJ, Muller WE. Piracetam reverses hippocampal membrane alterations in Alzheimer’s disease. J Neural Transm 1999;106:757-761.
20. El-Hazmi MAF, Al Fawaz I, Warsy AS, Opawoye AD, Abu Taleb H, Howsawi Z. Piracetam for the treatment of sickle cell disease in children — a double-blind test. Saudi Med J 1998;19:22-27.
21. Fassoulaki A, Kostopanagiotou G, Kaniaris P, Varonos DD. Piracetam attenuates the changes in the surface potential of the phosphatidylcholine monolayer produced by alcohols. Acta Anaesthesiol Belg 1985;36: 47-51.
22. Fedi M, Reutens D, Dubeau F, Andermann E, D’Agostino D, Andermann F. Long-term efficacy and safety of piracetam in the treatment of progressive myoclonus epilepsy. Arch Neurol 2001 ;58:781-786.
23. Flicker L, Grimley Evans J. Piracetam for dementia or cognitive impairment (Cochrane Review). In: The Cochrane Library, Issue 2. Chichester, UK: John Wiley & Sons Ltd., 2004.
24. Gianello P, Janssen T, Chatzopoulos C, et al. Beneficial effect of piracetam on renal blood flow in ischemi-cally injured kidneys in the rat. Transplant Proc 1988;20:914-916.
25. Giurgea C. Vers une pharmacologie de l’activite integrative du cerveau. Tentative du concept nootrope en psychopharmacologie. [Towards an integrative pharmacology of the activity of the brain. Attempt at the nootropic concept in psychopharmacology]. Actual Pharmacol (Paris) 1972;25:115-176.
26. Gobert JG. Genese d’un medicament: le piracetam. Metabolisation et recherche biochimique. [Genesis of the drug piracetam. Metabolism and biochemical research]. JPharm Belg 1972;27:281-304.
27. Gobert JG, Baltes EL. Availability and plasma clearance of piracetam in man. Farmaco 1977;3:84-91.
28. Gualtieri F, Manetti D, Romanelli MN, Ghelardini C. Design and study of piracetam-like nootropics, controversial members of the problematic class of cognition-enhancing drugs. Curr Pharm Des 2002;8:125-138.
29. Guidetti G, Galetti G. Valutazione clnica dell’influenza del piracetam sui fenomeni di adattamento centrale nelle vestibolopatie trattate e non con rieducazione oculomotoria. [Clinical evaluation of the influence of piracetam on the occurrence of central adaptation in vestibulopathies with or without oculomotor reeducation]. Riv Orl Aud Fond 1991;2:148-156
30. Hakkarainen H, Hakamies L. Piracetam in the treatment of post-concussional syndrome. A double-blind study. Eur Neurol 1978; 17:50-55.
31. Hawkins CA, Mellanby JH. Piracetam potentiates the antiepileptic action of carbamazepine in chronic experimental limbic epilepsy. Acta Neurol Scand 1986;74(Suppl 109): 117-121.
32. Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med 1980;302:992-995.
33. Herrmann WM, Kern U. Nootropika: Wirkungen und Wirksamkeit — Eine Uberlegung am Beispiel einer Phase III-Priifung mit Piracetam. [Nootropic drugs — effects and therapeutic efficacy: A phase III study with piracetam as a model]. Nervenarzt 1987;58:358-364.
34. Herrmann WM, Stephan K. Moving from the question of efficacy to the question of therapeutic relevance: An exploratory reanalysis of a controlled clinical study of 130 inpatients with dementia syndrome taking piracetam. Int Psychogeriatr 1992;4:25-44.
35. Herrschaft H. The effect of piracetam on global and regional cerebral blood flow in acute cerebral ischemia of man. Med Klin 1978;73:195-202.
36. Hoover R, Rubin R, Wise G, Warren R. Adhesion of normal and sickle erythrocytes to endothelial mono-layer cultures. Blood 1979;54:872-876.
37. Ikeda A, Shibasaki H, Tashiro K, Mizuno Y, Kimura J. Clinical trial of piracetam in patients with myoclonus: Nationwide multiinstitution study in Japan. The Myoclonus/Piracetam Study Group. Mov Disord 1996; 11:691-700.
38. Israel L, Melac M, Milinkevitch D, Dubos G. Drug therapy and memory training programs: A double-blind randomized trial of general practice patients with age-associated memory impairment. Int Psychogeriatr 1994;6:155-170.
39. Karacostas D, Doskas T, Artemis N, Vadicolias K, Milonas I. Beneficial effect of piracetam monotherapy on post-ischaemic palatal myoclonus. JInt Med Res 1999;27:201-205.
40. Kenny MW, Meakin M, Worthington DJ, Stuart J. Erythrocyte deformability in sickle-cell crisis. Br J Haematol 1981;49:103-109.
41. Koskiniemi M, Van Vleymen B, Hakamies L, Lamusuo S, Taalas J. Piracetam relieves symptoms in progressive myoclonus epilepsy: A multicentre, randomised, double-blind, crossover study comparing the efficacy and safety of three doses of oral piracetam with placebo. J Neurol Neurosurg Psychiatry 1998;64: 344-348.
42. Kruk Z. L, Pycock CJ. Neurotransmitters and Drugs. 3rd Edition. London: Chapman & Hall, 1993; 159-166.
43. Kulkami SK, Jog MV. Facilitation of diazepam action by anticonvulsant agents against picrotoxin induced convulsions. Psychopharmacology (Berl) 1983;81:332-334.
44. Levi G, Sechi E. A study of piracetam in the pharmacological treatment of learning disabilities. In: Child health and development, developmental dyslexia and learning disorders. Vol. 5. Bakker D, Ed. Basel: Karger, 1987;129-139.
45. Maass B, Soetanto R. Priifung der Wirkung von Piracetam auf die Wasserstoff— Clearance am Innenohr [Examination of the effect of piracetam on the hydrogen clearance to the inner ear]. Laryngorhinootologie 1988;67:132-135.
46. Mikati MA, Solh HM, Deryan DE, Sahli IF, Dabbous IA. A preliminary report on piracetam in sickle cell anemia: A double-blind crossover clinical trial and effects on erythrocyte survival. The King Faisal Spec Hosp J 1983;3:233-234.
47. Mingeot-Leclercq M-P, Lins L, Bensliman M, et al. Piracetam inhibits the lipid-destabilising effect of the amyloid peptide A(3 C-terminal fragment. Biochim Biophys Acta 2003;1609:28-38.
48. Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976;263:663-665.
49. Moncada S, Herman AG, Higgs EA, Vane JR. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. ThrombRes 1977;11:323-344.
50. Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin Pgl2), a potent inhibitor of platelet aggregation. Lancet 1977;1:18-20.
51. Mondadori C, Schmutz M. Synergistic effects of oxiracetam and piracetam in combination with antiepileptic drugs. Acta Neurol Scand 1986;74(Suppl 109): 113-116.
52. Mondadori C, Schmutz M, Baltzer V. Potentiation of the anticonvulsant effects of antiepileptic drugs by “nootropics”; a potential new therapeutic approach. Acta Neurol Scand 1984;69:131-132.
53. Moriau M, Crasbom L, Lavenne-Pardonge E, Von Frenckell R, Col-Debeys C. Platelet anti-aggregant and rheological properties of piracetam. Arzneimittelfors chung 1993;43:110-118.
54. Muller WE. Age related quantitative and qualitative receptor changes and pharmacological reactivity. In: Racagni G, Mendlewicz J, Eds. Treatment of age-related cognitive dysfunction: Pharmacological and clinical evaluation. Int Acad Biomed Drug Res. Basel: Karger 1992;2:35-40.
55. Muller WE, Eckert GP, Eckert A. Piracetam: Novelty in a unique mode of action. Pharmacopsychiatry 1999;32(Suppl l):2-9.
56. Muller WE, Koch S, Scheuer K, Rostock A, Bartsch R. Effects of piracetam on membrane fluidity in the aged mouse, rat and human brain. Biochem Pharmacol 1997;53:135-140.
57. Nalbandian RM, Henry RL, Burek CL, et al. Diminished adherence of sickle erythrocytes to cultured vascular endothelium by piracetam. Am JHematol 1983; 15:147-151.
58. Obeso JA, Artieda J, Quinn M, et al. Piracetam in the treatment of different types of myoclonus. Clin Neuro-pharmacol 1988;11:529-536.
59. Obeso JA, Artieda J, Rothwell JC, Day B, Thompson P, Marsden CD. The treatment of severe action myoclonus. Brain 1989;112:765-767.
60. Obeso JA, Rothwell JC, Marsden CD. The spectrum of cortical myoclonus. From focal reflex jerks to spontaneous motor epilepsy. Brain 1985; 108:193-224.
61. Olpe H-R, Steinmann MW. The activating action of vincamine, piracetam and hydergine on the activity of the noradrenergic neurons of the locus coeruleus. Behav Neural Biol 1981;33:249-251.
62. Oosterveld WJ. The efficacy of piracetam in vertigo. Arzneimittelforschung 1980;30:1947-1949.
63. Orgogozo JM. Piracetam in the treatment of acute stroke. Pharmacopsychiatry 1999;32(Suppl l):25-32.
64. Passeri M, Buonanno G, Lombardi C. Influenza del trattamento con piracetam sui sintomi cognitivi e com-portamentali di pazienti affetti da sdat. Trattamento con piracetam dei disturbi della cognitivea. [Influence of treatment with piracetam on cognitive and behavioural symptoms of patients with SDAT (senile dementia — Alzheimer type). Treatment of disturbed cognitive function with piracetam]. Arg Gerontol 1990;2:229-233.
65. Paula-Barbosa MM, Brandao F, Pinho MC, Andrade JP, Madeira MD, Cadete-Leite A. The effects of piracetam on lipofuscin of the rat cerebellar and hippocampal neurons after long-term alcohol treatment and withdrawal: A quantitative study. Alcohol Clin Exp Res 1991;15:834-838.
66. Peuvot J, Schank A, Deleers M, Brasseur R. Piracetam-induced changes to membrane physical properties. A combined approach by 31P nuclear magnetic resonance and conformational analysis. Biochem Pharmacol 1995;50:1129-1134.
67. Pilch H, Muller WE. Piracetam elevates muscarinic cholinergic receptor density in the frontal cortex of aged but not of young mice. Psychopharmacology 1988;94:74-78.
68. Rabbitt P, Lowe C. Patterns of cognitive ageing. Psychol Res 2000;63:308-316.
69. Reuse-Blom S. Microcirculation of the pial vessels in the rabbit. Acta Cardiol 1979;34:35-36.
70. Ricci S, Celani MG, Cantisani AT, Righetti E. Piracetam in acute stroke: A systematic review. J Neurol 2000; 247:263-266.
71. Ricci S, Celani MG, Cantisani AT, Righetti E. Piracetam for acute ischaemic stroke. Cochrane Database Syst Rev 2002;CD000419.
72. Rosenhall U, Deberdt W, Friberg U, Kerr A, Oosterveld W. Piracetam in patients with chronic vertigo. Clin Drug Invest 1996; 11:251-260.
73. Sato M, Heiss WD. Effect of piracetam on cerebral blood flow and somatosensory evoked potential during normotension and hypotensive ischemia in cats. Arzneimittelforschung 1985;35:790-792.
74. Scheuer K, Stoll S, Paschke U, Weigel R, Muller WE. N-methyl-D-aspartate receptor density and membrane fluidity as possible determinants of the decline of passive avoidance performance in aging. Pharmacol Biochem Behav 1995;50:65-70.
75. Schneider LS, Olin JT. Clinical global impressions in Alzheimer’s clinical trials. Int Psychogeriatr 1996;8: 277-288.
76. Schror K, Link HB, Rosen R, Klaus W, Rosen P. Prostacyclin-induced coronary vasodilation. Interactions with adenosine, cyclic AMP and energy charge in the rat heart in vitro. Eur J Pharmacol 1980;64:341-348.
77. Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: A critical perspective. Mech Ageing Dev 2001; 122:1-29.
78. Shorvon S. Pyrrolidine derivatives. Lancet 2001;358:1885-1892.
79. Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin Aging Study (BASE). Psychol Aging 2003; 18:318-331.
80. Stoll L, Schubert T, Muller WE. Age-related deficits of central muscarinic cholinergic receptor function in the mouse: Partial restoration by chronic piracetam treatment. Neurobiol Aging 1992; 13:39—44.
81. Sun AY, Sun GY. Neurochemical aspects of the membrane hypothesis of ageing. Interdiscipl Top Gerontol 1979;15:34-53.
82. Terry AV Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 2003; 306:821-827.
83. Terwinghe G, Daumerie J, Nicaise C, Rossillon C. Effect therapeutique du piracetam dans un cas de myoclonies d’action post-anoxique. [Therapeutic effect of piracetam in a case of postanoxic action myoclonus]. Acta NeurolBelg 1978;78:30-36.
84. Valzelli L, Bemasconi S, Sala A. Piracetam activity may differ according to the age of the recipient mouse. Int Pharmacopsychiatry 1980;15:150-156.
85. Van Hout A, Giurgea D. The effects of piracetam in dyslexia. Approche Neuropsychol Apprent VEnfant (ANAE) 1990;3:145-152.
86. Van Vleymen B, Van Zandijcke M. Piracetam in the treatment of myoclonus: An overview. Acta Neurol Belg 1996;96:270-280.
87. Waegemans T, Wilsher CR, Danniau A, Ferris SH, Kurz A, Winblad B. Clinical efficacy of piracetam in cognitive impairment: A meta-analysis. Dement Geriatr Cogn Disord 2002;13:217-224.
88. Wilsher CR, Bennett D, Chase CH, et al. Piracetam and dyslexia: effects on reading tests. J Clin Psycho-pharmacol 1987;7:230-237.
89. Wurtman RJ, Magic SG, Reinstein DK. Piracetam decreases hippocampal acetylcholine levels in rats. Life Sci 1981;28:1091-1093.
90. Xerri C, Zennou-Azogui Y. Influence of the postlesion environment and chronic piracetam treatment on the organization of the somatotopic map in the rat primary somatosensory cortex after focal cortical injury. Neuroscience 2003;118:161-177.