Citicoline Improves Verbal Memory in Aging
Paul A. Spiers, PhD; Diane Myers, MA; Gail 5. Hochanadel, PhD; Harris R. Lieberman, PhD; Richard J. Wurtman, MD
Objective: To test the verbal memory of older volunteers given citicoline.
Design: A randomized, double-blind, placebo-controlled, parallel group design was employed in the initial study. After data analysis, a subgroup was identified whose members had relatively inefficient memories. These subjects were recruited for a second study that used a crossover design. The subjects took either placebo or citicoline, 1000 mg/d, for 3 months in the initial study. In the crossover study, subjects took both placebo and citicoline, 2000 mg/d, each for 2 months.
Subjects: The subjects were 47 female and 48 male volunteers 50 to 85 years old. They were screened for dementia, memory disorders, and other neurological problems. Of the subjects with relatively inefficient memories, 32 participated in the crossover study.
Main Outcome Measure: Verbal memory was tested at each study visit using a logical memory passage. Plasma choline concentrations were measured at baseline; at days 30, 60, and 90 in the initial study; and at day 60 of each treatment condition in the crossover study. Plasma choline concentrations and memory scores were analyzed using repeated-measures analysis of variance and covariance, followed by planned comparisons when appropriate.
Results: In the initial study, citicoline therapy improved delayed recall on logical memory only for the subjects with relatively inefficient memories. In the crossover study, the higher dosage of citicoline was clearly associated with improved immediate and delayed logical memory.
Conclusions: Citicoline therapy improved verbal memory functioning in older individuals with relatively inefficient memories. Citicoline may prove effective in treating age-related cognitive decline that may be the precursor of dementia.
(Arch Neurol. 1996;53:441-448)
From the Clinical Research Center (Drs Spiers,Hochanadel, and Wurtman and Ms Myers) and Department of Brain and Cognitive Sciences (Dr Wurtman), Massachusetts Institute of Technology, Cambridge: and US Army Research Institute of Environmental Medicine,Natick, Mass (Dr Lieberman).
CHOLINERGIC brain neurons play a central role in learning and memory.12 These functions can be disrupted by giving normal volunteers anticholinergic agents, such as scopolamine,5 4 and can be restored by acetylcholinesterase inhibitors.5‘7 It is unclear, however, whether the administration of choline, acetylcholines precursor, can improve human memory. In some studies, oral choline therapy produced only modest effects48; in another study, high doses of phosphatidylcholine, the principal dietary choline source, significantly reduced the number of trials required by normal subjects to learn a list of nonsense syllables.* This effect was greatest among subjects who had initially required more trials to learn a similar list. Otherwise normal individuals with relatively poor memory function may, therefore, be the best candidates for choline supplementation.
The purpose of the present study was to determine whether oral administration of citicoline (cytidine diphosphocholine) (Ferrer Intemacional, Barcelona, Spain), a metabolic intermediate that completely dissociates to choline and cytidine on entering the body,10 improves memory in wellfunctioning older subjects. Besides promoting acetylcholine biosynthesis, this drug may also enhance synaptic transmission by facilitating the formation of neural membranes. Choline and cytidine, acting in concert, enhance phosphatidylcholine synthesis in cultured cells,11 rat brain slices,12 and whole brain in vivo,13 and this increase is followed by proportionate changes in levels of the other major membrane phospho-
SUBJECTS AND METHODS
SUBJECTS
The total study population was composed of 95 older subjects (47 women and 48 men) who were recruited through print advertisements or from a population that had previously participated in studies at the Clinical Research Center (CRC) of the Massachusetts Institute of Technology, Cambridge. Exclusion criteria precluding participation in this study included active medical, neurological, or psychiatric illness or a history of any condition that might significantly influence performance on cognitive testing.
Subjects were screened by medical history, physical examination, blood work, and electrocardiogram as well as by single photon emission computed tomography; all findings and values had to fall within normal limits for the subject’s age. Subjects also had to score 26 or greater (out of a possible score of 30, which is considered normal) bn the Mini-Mental State Examination. The Vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised was administered to assess general intelligence, with a score at the 25th percentile (average range) or better required. The Logical Memory subtest of the Wechsler Memory Scale and the Rey-Osterreith Complex Figure Test were administered to assess verbal and nonverbal learning; scores on these measures also had to be at least in the average range for age (see Lezak30 for descriptions of ail of these tests).
Informed consent was obtained from each subject after the experimental procedures and risks associated with the consumption of citicoline had been fully explained. Subject recruitment, informed consent procedures, and all experimental methods were approved by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects. Citicoline is an Investigational New Drug approved for human research by the Food and Drug Administration. Subjects were monitored closely for adverse experiences and encouraged to report any health complaints. A study physician was available on 24-hour call. Adverse experiences were recorded on case report forms, and subjects were followed up until such adverse experiences resolved or the subject was dropped from the study. Any subject dropped as a result of an adverse experience or for noncompliance was debriefed according to the guidelines of the Committee on the Use of Humans as Experimental Subjects. Any adverse event of sufficient severity to result in a subject being dropped from the study and believed to be related to treatment with the experimental compound was to be reported to the Food and Drug Administration. No such event occurred.
Subjects were assigned to either the placebo or active drug condition according to a blind randomization schedule that was applied to successively recruited subjects without regard to age, education, or baseline performance on screening measures. One subject was dropped for noncompliance during screening, and those data were not included in the baseline comparisons (n=94). The age and educational characteristics of the 2 experimental groups and their baseline performance on the criterion measure are presented in Table I. Subjects ranged in age from 50 to 85 years, with a mean (±SD) age of 67.2 (±9.3) years. There was no significant difference in the mean age of male (67.9±8 2 years) vs female (66.4± 10.3 years) subjects or in the mean age of subjects randomly assigned to the placebo group (67.9±9.5 years) vs the citicoline group (66.2±9.0 years). The sample was well educated, with a mean of 14.3 years of schooling. Men had significantly (t=2.23, PC.03) more years of schooling (15.3± 2.7 years) than women (14.1 ±2.6 years). Subjects assigned by the randomization process to the placebo group had significantly (t= — 2.28, PC .02) more years of education (15.3 ±2.7 years) than subjects assigned to the citicoline group (14.1 ±2.6 years). There was no drug group-by-sex interaction when these data were subjected to analysis of variance (ANOVA), however, since the mean years of education of the men and women in each drug group were not significantly different. There were no significant differences in baseline plasma choline concentrations between men and women or between the citicoline and placebo groups. There were no significant differences between baseline performances on immediate and delayed logical memory.
Baseline performance on a logical memory passage was used to classify subjects as having relatively inefficient memories. As described above, the sample was stratified into age groups, and the mean (SD) performance for each age group at baseline was calculated for immediate recall. Based on whether they scored below the mean relative to their peers, the subjects were then classified as having either average or relatively inefficient memories. Forty-nine subjects (22 in the citicoline group, 27 in the placebo group) were classified as having relatively inefficient memories based on these criteria, and this group’s performance was still within the normal range on this task.
A total of 32 subjects were recruited from this pool who were willing and able to participate in the relatively inefficient memory crossover study, 16 from the group assigned by the initial randomization to drug treatment and 16 from the group assigned to the placebo condition. The ages and educational characteristics of these 2 groups and their baseline performances on the criterion measures are presented in Table 1. Two-tailed t tests and analyses of variance did not reveal any significant comparisons or interactions between these 2 groups by gender, age, or years of education. These subjects ranged in age from 54 to 86 years, with a mean of 73.1 years. These groups, like the initial sample, were well educated, with 14.3 mean years of schooling.
There were no significant differences in baseline plasma choline concentrations between men and women (t=-0.23, P<.83) or between the citicoline and placebo groups (t=0.92, P<.36). There was no drug group-by-sex interaction. There was no significant difference between men and women in their baseline performance on logical memory (t=1.77, P<09). Similarly, there was no significant difference between the citicoline and placebo subgroups in their baseline performance on logical memory (£=0.71, P< 48). There was no drug group-by-sex interaction.
PROCEDURE
Citicoline and placebo tablets were formulated by Ferrer Intemacional SA in Barcelona, Spain. Tablets were identical in appearance, taste, and packaging. Once accepted into the protocol, all subjects took 1 tablet of placebo twice daily for 1 week prior to baseline studies to become acclimated to the experimental regimen. Subjects were then assigned to either the citicoline or placebo group according to the randomization schedule and were supplied with either placebo or citicoline tablets (500 mg), which they took twice daily for 3 months. If they continued in the crossover study, subjects were supplied with either placebo or citicoline tablets at the beginning of each treatment condition. They took 2 tablets twice daily for 2 months during each condition, with a 10-day washout period prior to crossing over.
Subjects were required to spend 1 morning per month at the CRC, beginning at the end of the initial placebo week, for a total of 4 visits (baseline and days 30, 60, and 90) in the initial study and 5 more visits (days 30 and 60, washout, and days 30 and 60) in the crossover study. At these monthly visits, vital signs were measured, health histories were recorded, and blood samples were taken by CRC nursing staff who were blind to treatment assignment. After their nursing visit, at baseline, day 30, and day 90 in the initial study and at day 60 of each condition in the crossover study, a battery of cognitive tests was administered by a trained technician who was also blind to treatment assignment.
MEASURES
Subjects had fasting blood samples drawn, consumed a normal breakfast, and then took their morning tablet; approximately 2 hours later, second blood samples were obtained. Laboratory tests performed included urinalysis, complete blood cell count, platelet count, and partial thromboplastin time, and the following concentrations were measured: blood glucose, serum urea nitrogen, creatinine, electrolytes, calcium, phosphorus, plasma proteins, bilirubin, alkaline phosphatase, aspartate aminotransferase, amylase, and creatine kinase. Plasma was also obtained and frozen for later assay to determine choline concentrations.
The verbal memory task was administered at the baseline, day 30, and day 90 visits in the initial study and at both day 60 visits in the crossover study. A narrative passage was read aloud to the subject once, followed by the subject’s immediate oral recall. Approximately 30 minutes later, delayed recall was measured. The Logical Memory subtest stories of the Wechsler Memory Scale and the Wech-sler Memory Scale-Revised, which contained the same number of target bits of information, were used at the various testing sessions. Alternate forms were used at the different study visits, but the same form was used for all subjects at each specific study visit. For example, all subjects heard the American Liner story at baseline and the Police Dogs story at day 30. All tests were administered and scored using standard criteria by the same examiner, who remained blind to treatment conditions. Scoring and data entry were verified by the principal investigator, who was also blind to treatments, and initial data analyses were conducted before the treatment assignment code was broken.
STATISTICAL ANALYSIS
Four subjects were dropped from the initial study as a result of adverse experiences (see below) and 1 subject for noncompliance during screening; data from the remaining 90 subjects were included in the statistical analysis. Choline assay results for all remaining subjects in each drug group (44 in the citicoline group, 46 in the placebo group) were compared by 2-factor (timeXdrug) repeated-measures ANOVA, followed by planned comparisons (1-tailed (tests). This analysis was used to determine whether there were any significant differences in plasma choline concentrations between the 2 experimental groups at days 30, 60, and 90. Performance on immediate and delayed logical memory was also compared at days 30 and 90 by repeated-measures analysis of covariance (ANCOVA), with baseline performance controlled to account for the significant difference in years of education between the experimental samples in this parallel group design. Two-factor repeated-measures ANCOVAs were also applied to the memory data of subjects classified in the relatively inefficient memory groups, followed by planned comparisons (1-tailed £ tests).
For the crossover study, plasma choline assays were compared by 2-factor (time Xgroup) repeated-measures ANOVA, followed by planned comparisons (1-tailed £ tests). This analysis was used to determine whether there were any significant differences in plasma choline concentrations between the 2 groups at washout and day 60 during either condition. Performance on immediate and delayed logical memory was compared by charting the subjects’ performance at baseline and day 90 of the initial study and at day 60 during each condition of the crossover study using 2-factor (timeXgroup) repeated-measures ANOVAs. If there was a significant group-by-time interaction, single-factor (time) ANOVAs were used to compare the performance of each group at baseline and during each treatment condition, followed by planned comparisons (Student-Newman-Keuls test).
In addition, citicoline may enhance dopaminergic neurotransmission.14 This action could also facilitate memory improvement, given that the arousal and attention components of this function depend on catechol-aminergic systems.14 Oral dose tolerance and pharmacokinetic studies suggest that citicoline is well tolerated and safe,15’18 producing only infrequent, minor side effects, even during 15 months of daily administration.19 Citicoline has been reported to accelerate recovery from stroke20 and from traumatic brain injury.1921’23 In a 1989 study, Agnoli et al24 tested the potential of this compound for improving memory in elderly patients (mean age, 74 years) complaining of mild to moderate memory problems. While the authors did not clearly define their criteria for memory impairment, the mean Mini-Mental State Examination score for their sample was 20.1 (SD, 3.25). In the standardization research for the Mini-Mental State Examination, a score of 20 or less was found to be consistent with dementia, delirium, or affective disorder but not with normal aging.25 After receiving citicoline, 1000 mg/d, or placebo for 3 and 6 weeks, subjects were tested using the Randt Memory Test, a measure that was specifically designed for longitudinal pharmacological studies.26
The results were considered promising, with citicoline -treated subjects showing a specific, statistically significant improvement in acquisition efficiency, although they did not differ from placebo-treated subjects in overall memory performance.
The present studies were designed to extend the initial findings of Agnoli et al24 in a population of normal older volunteers using a more widely available standardized measure of memory function: passages from the Logical Memory subtest of the Wechsler Memory Scale and the Wechsler Memory Scale-Revised.27 This task more closely resembles the memory requirements of real life, as the subject is read a selected passage aloud and then asked to repeat it, as opposed to being drilled repeatedly on a list of related or unrelated words. This task is also more like the demands of normal human memory, in that subjects are asked to recall what they have heard and then, after a delay, to retrieve it.
Although a few of our subjects reported mild forgetfulness, none complained of malignant memory disorders or showed evidence of memory impairment on screening examinations. Furthermore, none met the operational criteria for age-associated memory impairment (complaint of poor memory and objective evidence of memory performance 1 SD or more below the mean for young adults).28 Subtypes of memory changes associated with senescence other than age-associated memory impairment have been proposed,29 including age-consistent memory impairment (test performance within 1 SD below the mean for the patient’s age group) and late-life forgetfulness (test performance >1 SD below the mean for the patient’s age group). In DSM-/V (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), it is proposed that the term age-related cognitive decline be used to classify such conditions; however, no specific criteria are proposed, and no specific definition is provided for the memory-re la ted changes. However, any subject whose performance was below the mean for his or her peers might be considered to have a relatively inefficient memory. Consequently, once the data from the entire sample had been analyzed, we tested the hypothesis that subjects with normal but relatively less efficient memory were more likely to benefit from choline supplementation.
Relatively inefficient memory was defined as a performance score that fell below the mean for the subject’s peers within our sample. Subjects with relatively inefficient memory were identified as follows: First, the sample was stratified into 3 age groups (50 to 64, 65 to 74, and 75 to 85 years). Second, the mean (SD) performance for each age group at baseline was calculated for immediate recall. Third, subjects were classified as having either average or relatively inefficient memory, based on whether they scored below the mean relative to their peers. The logical memory data were then analyzed separately for the relatively inefficient memory group.
RESULTS
ADVERSE EXPERIENCES
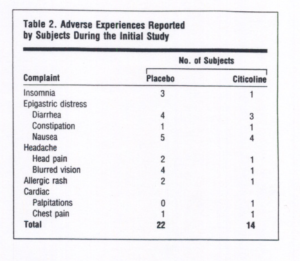
Subjects were encouraged to report any health complaints to the CRC nursing staff and were queried for adverse symptoms during their monthly visits. Forty such complaints or adverse experiences were reported in the initial study. Of these, 4 were clearly the result of concurrent medical problems unrelated to the subjects’ participation in the protocol (eg, cerebral aneurysm). These subjects were dropped from the study, leaving a total of 36 adverse experiences reported by 90 subjects during 3 months receiving either placebo or citicoline. These complaints were grouped into categories, including insomnia, epigastric distress (diarrhea, constipation, nausea), headache (head pain, blurred vision), rash, and cardiac complaints (palpitations, chest pain), none of which occurred with significantly greater frequency among citi-coline-treated subjects. In fact, the total number of adverse experiences in the initial study was greater among subjects receiving placebo than among those receiving citicoline (Table 2).
In the crossover study, only 9 adverse experiences were reported by the 32 subjects. Of these, 3 were clearly the result of concurrent medical problems unrelated to the subjects’ participation in the protocol (eg, macular degeneration). These subjects were dropped from the study, leaving a total of 6 adverse experiences reported by 29 subjects in 6 months. These 6 adverse experiences included 9 symptoms that could be grouped according to the categories used for the initial study. There were 4 episodes of epigastric distress, 2 of rash, 2 of headache, and I of insomnia. The insomnia episode occurred during the placebo period, but all others took place during citicoline treatment. Two other subjects also had to be dropped from the analysis of the crossover study results. One subject was dropped for incomplete data because of noncompliance during cognitive testing at his last visit. The other subject was dropped because a brain tumor was diagnosed shortly after he completed participation in the crossover study. This left 27 crossover subjects (14 in the citicoline group, 13 in the placebo group) from whom data could be used for treatment analyses. In summary, no adverse events were judged by CRC physicians to be related to citicoline treatment in either the initial study or the crossover study that required medical intervention, termination from the study, or an emergent report to the Food and Drug Administration.
CHOLINE
Repeated-measures ANOVA of plasma choline concentrations yielded a significant main effect for drug (F=7.71, PC.007) in the initial study. There was no main effect for time comparing results over days 30,60, and 90, and the interaction term was not significant. Planned comparisons (1-tailed t tests) showed that at each study visit, citicoline-treated subjects had significantly higher mean plasma choline concentrations than placebo-treated subjects (t=2.26, PC.02; t=0.77, PC.04; and 1=1.97, PC.03 at days 30, 60, and 90, respectively).
In the crossover study, repeated-measures ANOVA of plasma choline concentrations comparing placebo with citicoline yielded a significant main effect by drug (F=51.82, PC.001). There was no significant subgroup-by-drug interaction (citicoline vs placebo) and no group-by-drug interaction. Planned comparisons showed that citicoline treatment resulted in much higher plasma choline concentrations than placebo treatment for both groups of subjects (1=2.26, PC.001).
LOGICAL MEMORY
Repeated-measures ANCOVAs for all subjects in the initial study yielded a main effect for time on both the immediate (F=5.22, PC.03) and delayed (F=7.08, Pc.01) logical memory tasks. There was no main effect for drug, nor was there a drug-by-time interaction. The main effect for time showed that all subjects improved their performance compared with baseline.
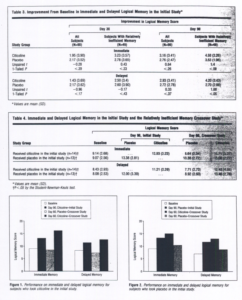
For the relatively inefficient memory group in the initial study, repeated-measures ANCOVAs yielded no main effects and no interaction effects on the immediate logical memory task. On delayed logical memory, there were no main effects, but a trend (F=2.78, PC. 10) was observed toward a drug-by-time interaction, suggesting that citicoline-treated subjects consistently improved in their performance compared with baseline when they were tested at days 30 and 90. Planned comparisons applied to improvement in recall from baseline scores revealed that this trend was caused by a significant difference in favor of citicoline for delayed logical memory at day 90 (t=1.68, PC.05). These data regarding performance improvement for the whole sample vs the relatively inefficient memory group on immediate and delayed logical memory at days 30 and 90 are presented in Table 3.
In the crossover study, 2-factor repeated-measures ANOVA of the 2 groups under each treatment condition showed a significant main effect of treatment condition (F=23.78, PC.001) and a significant group-by-treatment interaction (F=38.24, PC .001) for immediate recall. For delayed recall, 2-factor repeated-measures ANOVA also showed a significant main effect of treatment condition (F=9.98, PC.001) and a significant group-by-treatment interaction (F=18.05, PC.001). Single-factor repeated-measures ANOVAs showed a significant main effect for treatment condition in the citicoline (F=32.36, PC.001) and placebo (F=30.42, PC.001) subgroups on immediate recall as well as significant main effects of treatment condition in the citicoline (F=9.47, PC.001) and placebo (F=24.36, PC.001) subgroups on delayed recall. Planned comparisons revealed that for both the immediate and delayed recall tasks, the best performance of both the citicoline and placebo subgroups (PC.05) was during the citicoline treatment condition (Tabla 4, Figure 1, and Figure 2).
COMMENT
All subjects, regardless of experimental treatment, showed a practice effect, improving their verbal memory performance compared with the baseline. When the data were analyzed for the relatively inefficient memory group, there was no significant main effect for treatment, illustrating the importance of practice effects in such a repeated-measures design. There were statistical trends in the analysis, however, which planned comparisons showed were related to a significant difference on delayed recall performance. The subjects with relatively inefficient memory who received citicoline maintained their improvement from day 30 to day 90, whereas subjects who received placebo showed initial improvement at day 30, consistent with practice, but subsequently stayed the same or declined in performance by day 90. This finding suggested that the results observed with citicoline were caused by more than a simple practice effect.
When subjects with relatively inefficient memory were studied while receiving a higher dose of citicoline, verbal memory clearly improved. This effect was attributable to the citicoline, since performance was significantly lower when the same subjects were taking placebo. This suggests that the optimal dose of citicoline for producing cognitive effects in this population may be higher than was previously supposed, although still well within tolerance limits, given the absence of serious side effects observed in this and other studies.
This study was designed to test the earlier report by Agnoli et al24 that citicoline facilitates memory acquisition in the elderly. We did not observe such an effect in our overall population; however, this sample was higherfunctioning than that studied by Agnoli et al,24 according to their mean Mini-Mental State Examination scores. When we studied subjects with relatively inefficient memory1 2, we did find that citicoline facilitated memory’ acquisition and retention, confirming the findings of Agnoli et al. Since plasma choline concentrations were significantly higher for subjects when they were receiving citicoline, these findings confirm that subjects were compliant during the treatment condition and support the view that the drug s memory effect may result from changes in brain choline metabolism mediated by acetylcholine and phosphatidylcholine.
Overall, this study showed that citicoline improved verbal memory in elderly subjects whose performance was below that of their peers. Our test sample was composed of well-educated, functionally independent older adults. None of the subjects exhibited dementia, although a few might have met the criteria for age-associated memory impairment. Most of our subjects were performing above average for their age on this memory task at baseline. For those who were not performing as well as their high-functioning peers, however, results were dramatic in the relatively inefficient memory crossover study. The mean age of subjects with relatively inefficient memory was 6 to 8 years older than the mean age of the population for the initial study, with most of the former in their early 70s. The mean level of education of the subjects with relatively inefficient memory, however, was not different from that of the initial study population. Thus, the finding that verbal memory was improved by the dose of citicoline used in the crossover study suggests that this compound may be useful in the treatment of individuals who experience reduced memory functioning with advancing age. While certain authors31 have suggested that this should be viewed as a normal, inevitable consequence of senescence, others32 have maintained that age-related changes in cognitive function should no more be tolerated than declining visual acuity observed with aging and should not remain untreated.
This study suggests that citicoline should also prove helpful for elderly who are more typical intellectually than the sample described here. Furthermore, citicoline may provide considerable benefit to older individuals with age-associated memory impairment or other symptoms of mild cognitive impairment—symptoms that may herald the onset of progressive cognitive decline and may in fact be the precursors of dementia.33 Research to discover those abilities that would be most predictive of such impending progressive decline33 identified verbal memory. While the verbal memory task identified differed from that used in the present study, it remains to be determined whether citicoline could improve performance on verbal memory tasks other than logical memory, specifically for patients experiencing mild cognitive impairment. To the extent that citicoline can reverse such impairments and may delay the onset of such dementing conditions, further research should be conducted with this compound in patients who are experiencing memory’ loss or other forms of mild cognitive impairment.
Accepted for publication February 16, 1996.
This work was supported in part by a grant from the Center for Brain Sciences and Metabolism Charitable Trust, Cambridge, Mass, by grant MH-28783 from the National Institutes of Health, Bethesda, Md, and by grant MOl-RR-00088 from the National Institutes of Health to the CRC of the Massachusetts Institute of Technology, Cambridge.
The authors thank John Growdon, MD, John Gabri-elli, PhD, Andrew 5a£iin, PhD, Donald Schomer, MD, Keith Johnson, MD, William Abend, MD, Antoine El-Khoury, MD, and David August, MD, as well as Susan Blackburn, research assistant, Pauline Marchant, RN, nursing coordinator, and the CRC Nursing and Dietary Staff for assistance in carrying out this study. The authors also thank Jess Torres-Herrera, MD, and J’ Alfonso Ortiz-Hemandez, MD,/or their critical reading of the manuscript.
Reprints: Paul A. Spiers, PhD, CRC: E17-438, Massachusetts Institute of Technology, 77 Massachusetts Ave, Cambridge, MA 02139.
REFERENCES
Novel Biochemical. Pharmacological, and Clinical Aspects of CDP-Choline. New York. NY: Elsevier Science Publishing Co Inc; 1985:251-258
18 Zornelzer SF. Brain substrates of senescent memory decline. In: Squire L. Butters N. eds. Neuropsychology of Memory. New York, NY: Guilford Press; 1984: 588-600.
19. Lozano-Femandez R. Efficacy and safety of oral CDP-choline. Drug Res. 1983; 33:1073-1080.
20. Tazaki Y, Sakai F, Otomo E. et al. Treatment of acute cerebral infarction with a choline precursor in a multicenter double-blind placebo-controlled study. Stroke. 1988:19:211-216.
21. Catalyad Maldonado V, Catalyud Perez JB. Aso Escario J. Effects of CDP-choline on the recovery of patients with head injury. J Neurol Sci. 1991 ;103 (suppl):S15-S18.
22. Lozano R. CDP-choline in the treatment of cranio-cephalic traumata. J Neurol Sci. 1991 ;103(suppl):S43-S47.
23. Levin HS. Treatment of postconcussional symptoms with CDP-choline, J Neurol Sci. 1991;103(supp!):S39-S42.
24. Agnoli A, Bruno G, Fioravanti M. Therapeutic approach to senile memory impairment: a double-blind clinical trial with CDP-choline. In: Wurtman RJ, Cor-kin S. Growdon JH, eds. Alzheimer’s Disease: Proceedings of the Fifth Meeting of the International Study Group on the Pharmacology of Memory Disorders
Associated With Aging. Boston. Mass: Birkhauser: 1989:649-654.
25. Folstein MF. Folstein SE. McHugh PR. Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198
26. Randt CT, Brown ER. Osborne DJ. Cited by: Lezak MD. ed. Neuropsychological Assessment. 2nd ed. New York, NY: Oxford University Press; 1983:469.
27. Wechsler Memory Scale: Wechsler Memory Scale-Revised. New York, NY: Har-court Brace Jovanovich-The Psychological Corp: 1987.
28. Crook TH, Bartus RT, Ferris SH, Whitehouse P, Cohen GO. Gershon S. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change—report of a National Institute of Mental Health work group. Dev Neuropsychol. 1986;2:261-276.
29. Blackford RC, La Rue A. Criteria for diagnosing age-associated memory impairment: proposed improvements from the field. Dev Neuropsychol. 1989;5; 295-306.
30. Lezak M. Neuropsychological Assessment. 2nd ed. New York, NY: Oxford University Press; 1983.
31. O’Brien JT, Levy R. Age associated memory impairment. BMJ. 1992;304:5-6.
32. Crook TH, Ferris SH. Age associated memory impairment. BMJ. 1992;304:714.
33. Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006-1009.
Bartus RT. Dean RL, Beer B. Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408-417,
2. Bartus RT, Dean RL, Pontecorvo MJ. Flicker C. The cholinergic hypothesis: a historical overview, current perspective, and future directions. Ann N Y Acad Sci. 1985;444:332-358.
3. Drachman D. Sahakian BJ Effects of cholinergic agents on human learning and memory. In: Barbeau S, Growdon JH. Wurtman RJ, eds. Nutrition and the Brain. New York, NY: Raven Press; 1979;5:351-366.
4. Sitaram N, Weingartner H, Giilin JC. Human serial learning: enhancement with arecholine and choline and impairment with scopolamine. Science. 1978;201: 274-276.
5. Rusted JM. Warburton DM. The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacoiogy 1988;96:145-152.
6. Meador KJ, Loring DW, Davis HC, et al. Cholinergic and serotonergic effects on the P3 potential and recent memory. J Clin Exp Neuropsychol. 1989,11: 252-260.
7. Molchan SE. Mellows AM, Lawlor BA, et al. TRH attenuates scopolamine-induced memory impairment in humans. Psychopharmacoiogy. 1990; 100:84-89
8 Sorgatz H. Effects of lecithin on memory and learning. In: Hanin I, Ansell G8, eds. Lecithin. Technological. Biological and Therapeutic Aspects: Proceedings of the Fourth International Colloquium on Lecithin. New York, NY: Plenum Press; 1987:147-153.
9. Ladd SL, Sommer SS. Phospatidylcboline enhances short-term memory in slow learners. Presented at the 95th annual meeting of the American Psychological Association; August 20. 1990; Boston, Mass.
10. Lopez G.-Coviella I. Agut J, Von Borstel R, Wurtman RJ. Metabolism of cyti-dine (5′)-diphosphocholine (Citicoline) following oral and intravenous administration to the human and the rat. Neurochem Int. 1987;11:293-297.
Lopez G.-Coviella I, Wurtman RJ. Enhancement by cytidine of membrane phospholipid synthesis. J Neurochem. 1992;59:338-343.
12. Savci V. Wurtman RJW. Effect of cytidine on membrane phospholipid synthesis in rat striatal slices. Brain Res. 1995;64:378-384.
13. Lopez G.-Coviella I, Agut J, Ortiz A, Wurtman RJ. Effects of orally-administered cytidine 5′-diphosphate choline on brain phospholipid content. J Nutr Biochem. 1992;3:313-315.
14. Fonlupt P, Martinet M, Pacheco H. Effect of CDP-choline on dopamine metabolism in central nervous system. In: Zappia V, Kennedy EP, Nilsson Bl, GaJ-etti P. eds. Novel Biochemical, Pharmacological, and Clinical Aspects of COP-Choline. New York, NY: Elsevier Science Publishing Co Inc; 1985:169-177.
15. Dinsdale JRM, Griffiths GK, Castello J, Ortiz JA, Maddock J, Aytward M. C-CDP-choline: repeated oral dose tolerance studies in adult healthy volunteers. Drug Res. 1983;33:1061-1065.
16. Dinsdale JRM. Griffiths GK, Rowlands C, et al. Pharmacokinetics of C-CDP-choline. Drug Res. 1983;33:1066-1070.
17. Canonico PL. Sortmo MA. Speciale C Phospholipids and prolactin secretion: the effect of CDP-choline. In: Zappia V. Kennedy EP. Nilsson Bl, Galetti P. eds.
rc i/jvrs Cinultitwn
The Archives is available by request to nonfederal physicians in the United States (50 states and Washington, DC) whose official American Medical Association masterfile record shows a primary specialty of neurology or child neurology in an office- or hospital-based practice as a staff physician, resident in training beyond the first year, or clinical fellow.
If you meet the above qualification criteria and are not currently receiving the Archives and would like to receive it each month, you must complete a free subscription request card. To receive a request card, please write to Kathryn Osten, American Medical Association, Circulation Processing Department, 515 N State St, Chicago, IL 60610 (FAX 312-464-5831). A subscription request card will be sent to you in response. If you are a resident or fellow, please include verification of your training program and a complete mailing address.